Image Credit: Medtronic
Things have gone well for Boston Scientific with its subcutaneous implantable cardioverter defibrillator (S-ICD). These devices have no transvenous leads, and as such provide a solution for patients at risk for SCD when implantation of a traditional ICD with transvenous leads is not possible or desired.
S-ICDs have their disadvantages though – current models are larger in size because of the high energy they must deliver to defibrillate from a subcutaneous lead (as a reference, Boston Scientific’s EMBLEM S-ICD delivers 80J shocks), and do not provide antitachycardia pacing (ATP) or bradycardia pacing support, except for limited post-shock pacing.
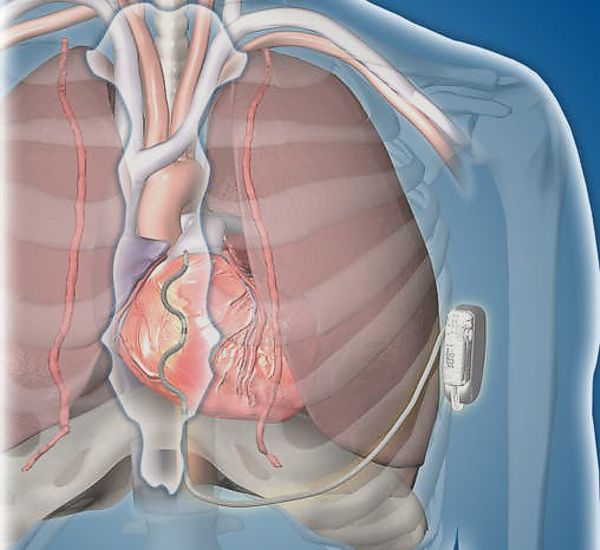
Medtronic is proposing a different approach to Boston Scientific’s. They call it the Extravascular Implantable Cardioverter Defibrillator (EV ICD), which uses a lead placed under the sternum, which gets the electrodes much closer to the heart than Boston Scientific’s subcutaneous lead.
An acute study using Medtronic’s lead configuration was published in JACC in 2018 and concluded
“The substernal lead was implanted in 79 patients, with a median SD implantation time of 12.0 ± 9.0 min. Ventricular pacing was successful in at least 1 vector in 76 of 78 patients (97.4%), and 72 of 78 (92.3%) patients had capture in 1 vector with no extracardiac stimulation. A 30-J shock successfully terminated 104 of 128 episodes (81.3%) of ventricular fibrillation in 69 patients.”
Mike Coyle, Executive VP and Group President of Medtronic’s Cardiac and Vascular Group had stated:
“Instead of placing the lead underneath the skin on top of the sternum, as is done with existing sub-Q systems, we would target putting the lead underneath the sternum and using a specialized introducer tool, would advance the lead in what is a very clear cavity in which to place a lead.”
Medtronic announced in August 2018 that the first EV ICD had been implanted in a patient.
Medtronic’s website: https://www.medtronic.com
