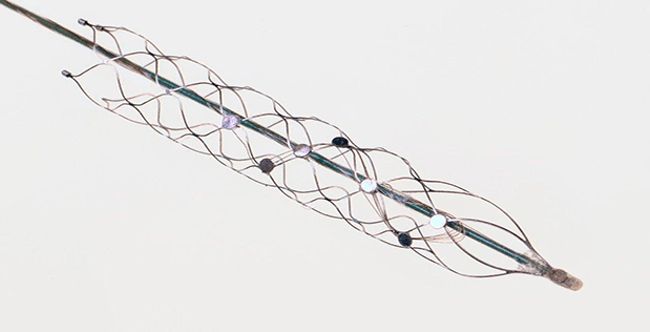
Image Credit: Synchron
Synchron Inc. announced today the first successful clinical implantation of the Stentrode®, a minimally-invasive neural interface technology, a component of the Synchron Brain-Computer Interface. This is the first clinical feasibility trial evaluating this technology for its potential to restore communication in people with severe paralysis.
Synchron is a Silicon Valley company that spun out of the University of Melbourne, Australia in 2012. It was started by Thomas Oxley, MD, PhD to develop a new concept for a Brain-Computer Interface
Synchron’s Stentrode™ is an endovascular neural interface. It is essentially an electrode array shaped as an endovascular stent that can be implanted via the jugular vein and advanced into the brain to the motor cortex. Neural signals are detected by the electrodes on the Stentrode™ and sent to a processing and communications unit implanted subcutaneously in the chest, and then wirelessly to an external receiver. The idea is that the device can interpret signals from the brain for patients with paralysis to control a computer operating system and set of applications that interact with assistive technologies.
According to the announcement:
“The Stentrode is the only investigational implantable device that does not require open brain surgery and is designed to record brain activity and stream thoughts wirelessly directly from the brain.
The technology relies on a revolutionary brain-controlled handsfree app platform called brainOS™ to translate the brain activity into a standardized digital language to control apps that restore communication and limb function. In addition, brainPort™, a fully-internalized, wireless solution implanted in the chest provides high-resolution neural data transmission, and is the final component of the Synchron Brain-Computer Interface.
…
The trial of the Stentrode in combination with brainOS software will evaluate the safety, as well as assess the stability of high-fidelity signals acquired from the brain to control external communications technologies. The trial is being conducted in Melbourne, Australia and will include patients with loss of motor function from paralysis due to a range of conditions including spinal cord injury, stroke, muscular dystrophy, or motor neuron disease (ALS) patients. “
Synchron’s website is at: https://www.synchronmed.com
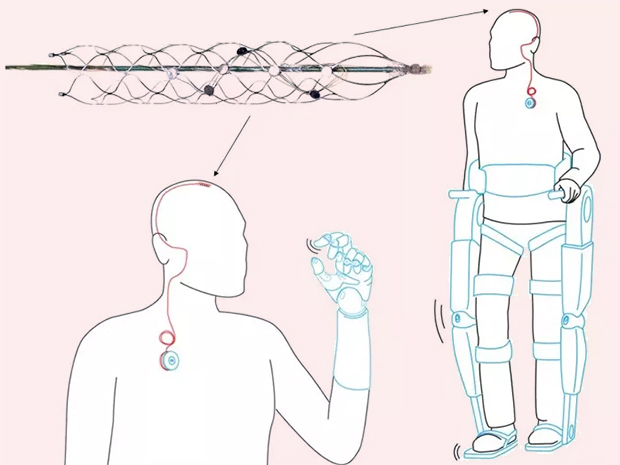
Conceptual use of the Stentrode device
Image Credit: University of Melbourne
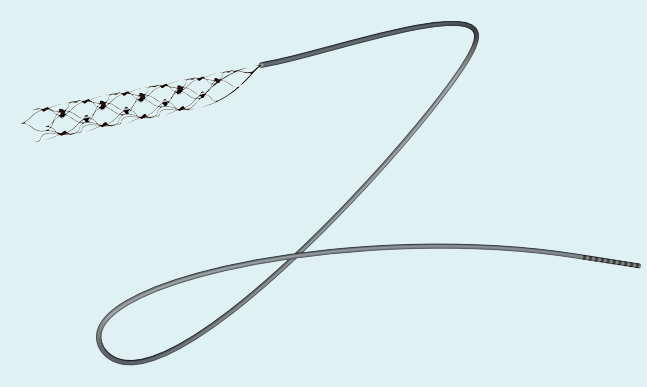
Synchron’s Stentrode®, a minimally-invasive neural interface
