Image credit: Impulse Dynamics
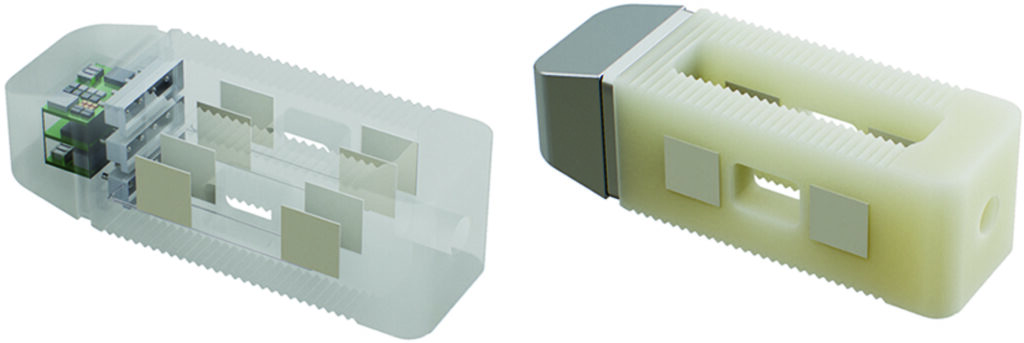
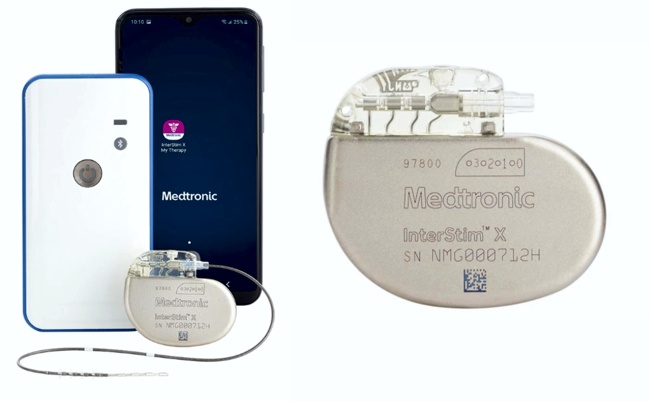
Impulse Dynamics, the company where I am the CTO and Executive VP of Product Development, announced on May 18, 2023 the completion of the first implantation for the INTEGRA-D clinical trial, designed to evaluate the safety and efficacy of two proven cardiac therapies combined — CCM® and an implantable cardioverter defibrillator (ICD) — in a single device (CCM-D). The Optimizer® IntegraTM CCM-D System delivers CCM therapy to improve quality of life and reduce heart failure symptoms, and ICD therapy to treat life-threatening arrhythmias that may cause sudden cardiac death. The investigational technology is rechargeable with long battery life, potentially reducing the need for replacement procedures.