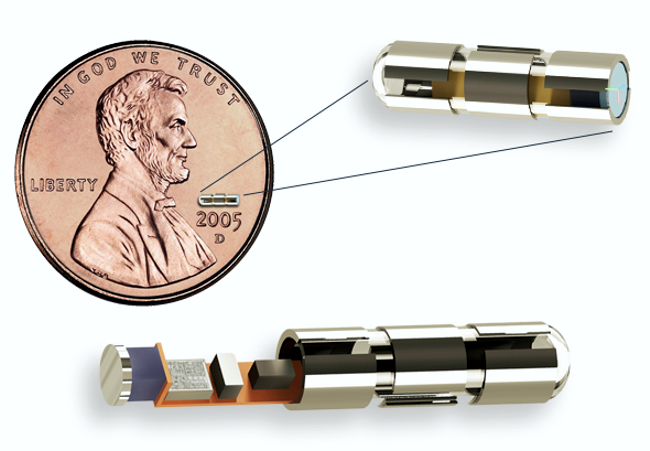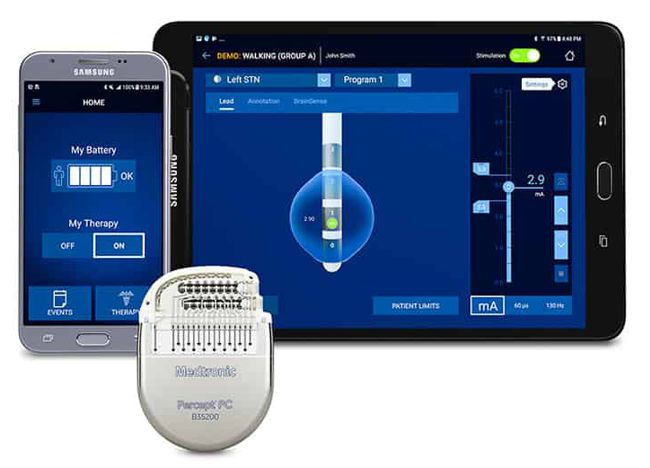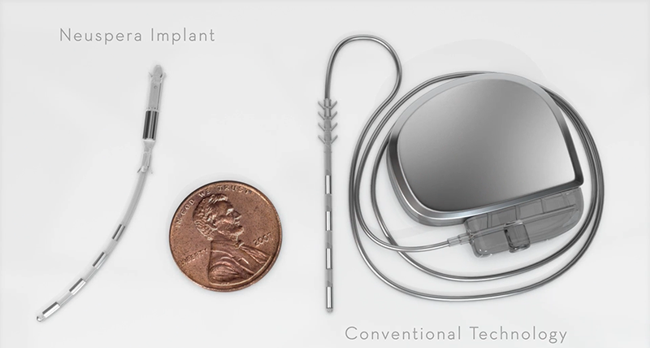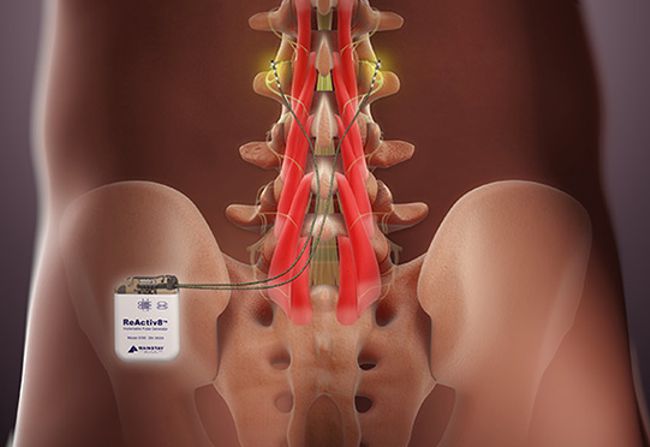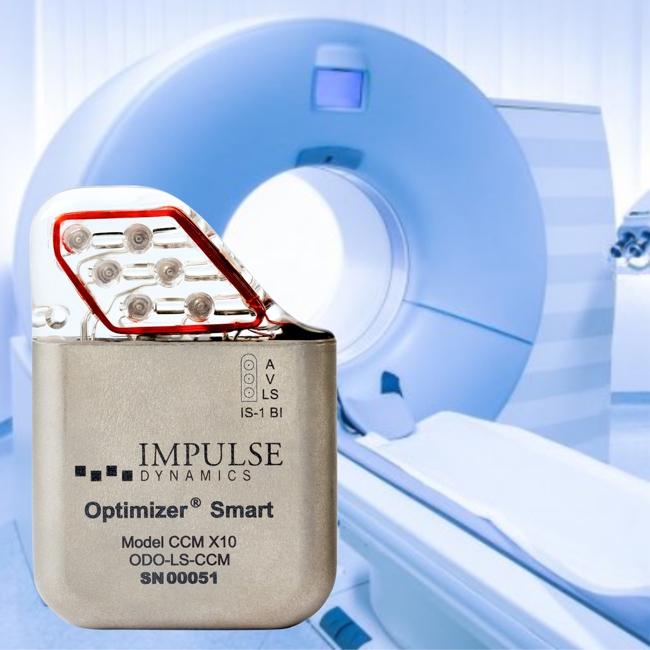
Impulse Dynamics – the company for which I am CTO and Executive VP – received MR-conditional approval (1.5 T head/limbs with peripheral coils) from both FDA and the European Union.
From the press release:
IMPULSE DYNAMICS ANNOUNCES FDA APPROVAL FOR MAGNETIC RESONANCE IMAGING FDA
Clears Potential Hurdle for Many Heart Failure Patients
MARLTON, N.J.–(BUSINESS WIRE)–Impulse Dynamics, a company dedicated to improving the lives of people with heart failure (HF), today announced the U.S. Food and Drug Administration (FDA) has approved the conditional use of Magnetic Resonance Imaging (MRI) for Optimizer® CCM® delivery systems. This approval represents a significant advance because the population that benefits most from cardiac contractility modulation therapy (patients with moderate to severe HF) often requires advanced diagnostic imaging procedures.