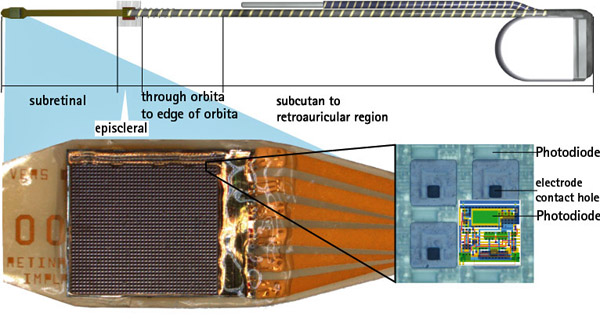
Retina Implant AG published study results on its Alpha IMS Implant for functional vision restoration. According to the press release, “Retina Implant AG, the leading developer of subretinal implants for patients blinded by retinitis pigmentosa (RP), announced results from part of its multicentre study were published today in the peer-reviewed journal Proceedings of the Royal Society B. The research found that, during the course of a three to nine month observation period, functional vision was restored in the majority of nine German patients implanted with a subretinal microchip as part of the first module of the Company’s second human clinical trial. In addition, visual acuity for two of the nine patients surpassed the visual resolution of patients from the Company’s first human clinical trial.