Retina Implant AG published study results on its Alpha IMS Implant for functional vision restoration. According to the press release, “Retina Implant AG, the leading developer of subretinal implants for patients blinded by retinitis pigmentosa (RP), announced results from part of its multicentre study were published today in the peer-reviewed journal Proceedings of the Royal Society B. The research found that, during the course of a three to nine month observation period, functional vision was restored in the majority of nine German patients implanted with a subretinal microchip as part of the first module of the Company’s second human clinical trial. In addition, visual acuity for two of the nine patients surpassed the visual resolution of patients from the Company’s first human clinical trial.
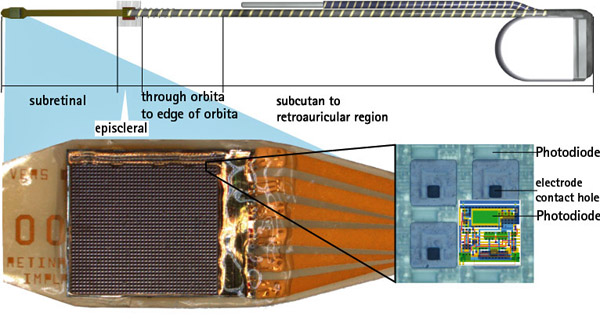
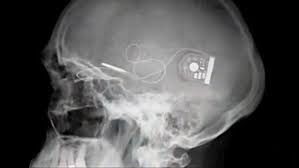
The study titled “Artificial Vision with Wirelessly Powered Subretinal Electronic Implant Alpha IMS” was led by researchers at the University Eye Hospital in Tuebingen, Germany including Professor Eberhart Zrenner and Katarina Stingl, M.D. Patients were implanted with Retina Implant AG’s subretinal wireless 3×3 mm2, 1500 pixel Alpha IMS microchip and are able to adjust the level of stimulation received to view objects at varied distances. Of the nine patients observed in the study, three patients were able to read letters spontaneously. During observation in and outside the laboratory patients also reported the ability to recognize faces, distinguish objects such as telephones and read signs on doors.
“The results of our first human clinical trial exceeded our expectations, and we are further encouraged by the results from the second human trial,” said Professor Eberhart Zrenner, M.D., lead clinical trial investigator, Institute for Ophthalmologic Research at University Eye Hospital Tuebingen, Germany. “As physicians, we are constantly seeking out the best treatment options for our most in-need patients, which most definitely includes those suffering from advanced-stage retinitis pigmentosa. This research provides additional evidence that our subretinal implant technology can help some patients with retinal degeneration regain functional vision and does so in a way that does not require externally visible equipment.”
“The continuation of our trial and publication of this module is the next milestone in our quest to secure CE mark approval and provide RP patients living in darkness with a treatment option,” said Walter-G Wrobel, president and CEO, Retina Implant AG. “We are continually humbled by the 36 patients we’ve implanted thus far and their willingness to participate in this ground-breaking research.”
The Company’s first clinical trial began in Germany in 2005, where 11 patients suffering from retinitis pigmentosa were implanted with a subretinal microchip below the retina in the macular region. Results from the first trial were published in November 2010 in Proceedings of the Royal Society B, concluding that the implantation of Retina Implant’s microchip was successful in restoring useful vision in patients previously blind due to retinitis pigmentosa. The second clinical trial with a wireless device that allows patients to use the implant outdoors and at home began in May 2010 in Tuebingen, Germany, and has since expanded into the multicentre phase of the trial with implants taking place in Hong Kong and the UK.
“As a leading relevant patient organisation in the UK we have been watching Retina Implant AG’s research with great interest,” said David Head, CEO of RP Fighting Blindness. “The results published today show definite promise to one day restore functional vision to patients with advanced-stage retinitis pigmentosa.”
UPDATE: April 2019: Retina Implant AG discontinues business activities
From the Retina Implant website:
“The shareholders of Retina Implant AG resolved to dissolve the company at an extraordinary general meeting held on 19 March 2019. There are two main reasons for this:
1. Our work has been hampered by the innovation-hostile climate of Europe’s rigid regulatory and health systems.
2. Whilst the majority of patients perceived their experience with our products as positive, the results achieved ultimately fell short of expectations.”
