Sorin announced it received U.S. Food and Drug Administration (FDA) conditional approval for its Investigational Device Exemption (IDE) application and clinical trial protocol for RESPOND CRT. The trial will study the safety and effectiveness of the innovative SonR cardiac resynchronization therapy (CRT) optimization system in patients with advanced heart failure. RESPOND CRT is a multi-center, randomized, two-arm, double-blinded, prospective trial that will enroll more than 1,000 patients in the United States and other geographies.
Sorin announced it received U.S. Food and Drug Administration (FDA) conditional approval for its Investigational Device Exemption (IDE) application and clinical trial protocol for RESPOND CRT. The trial will study the safety and effectiveness of the innovative SonR cardiac resynchronization therapy (CRT) optimization system in patients with advanced heart failure. RESPOND CRT is a multi-center, randomized, two-arm, double-blinded, prospective trial that will enroll more than 1,000 patients in the United States and other geographies.
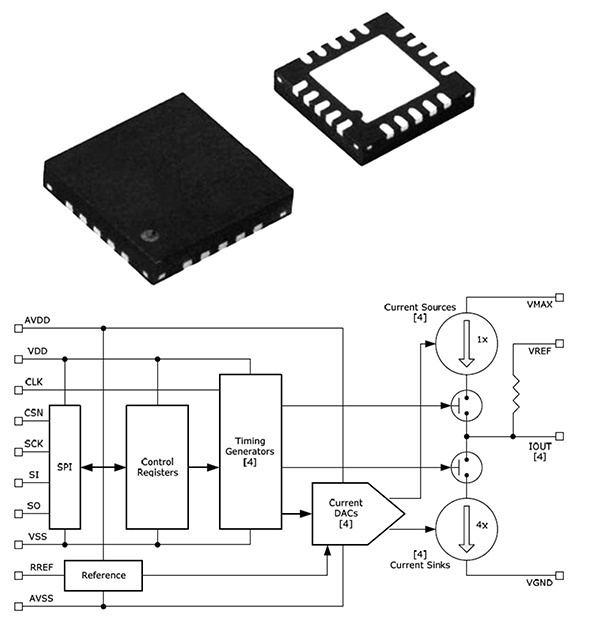
The SonR optimization system is comprised of the SonR hemodynamic sensor embedded in the SonRtip atrial pacing lead and the PARADYM RF SonR CRT-D device, which includes a unique algorithm to automatically optimize the patient’s atrioventricular (AV) delay and interventricular (VV) delay timing. While there are several CRT systems on the market, studies have shown that approximately one-third of patients with advanced heart failure do not effectively respond to CRT. The SonR system is the first and only CRT hemodynamic sensor based system designed to automatically adjust on a weekly basis. The timing of electrical impulses delivered to the heart is based on the patient’s heart activity and need – with the goal of improving the patient’s response to CRT. Typically this device re-programming is done manually in the clinic using echocardiography.

 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.