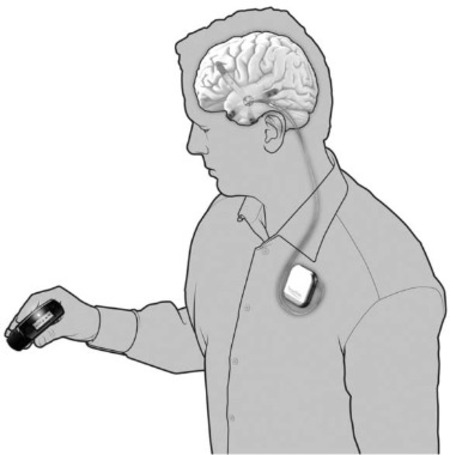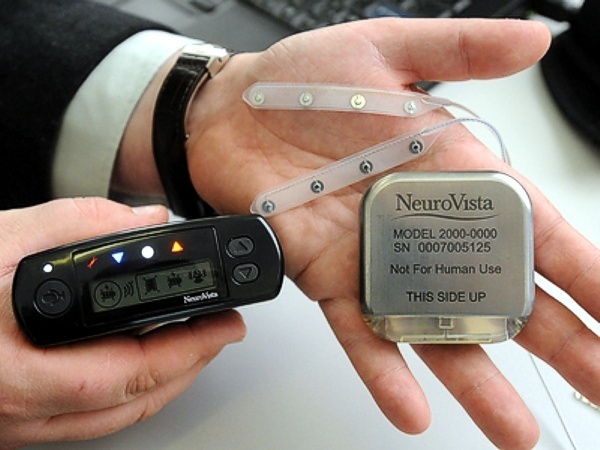
Seattle-based NeuroVista was founded in 2002 by Dr. Daniel DiLorenzo to develop an implantable device for the early detection of epileptic seizures. The NeuroVista seizure advisory system is based on an implantable device that senses EEG irregularities that precede a seizure. Early warning allows patients to take medicine and find a safe place to lie down. Although some epilepsy sufferers can feel seizures coming, many cannot.
In NeuroVista’s Seizure Advisory System (SAS), intracranial EEG signals are recorded through electrodes implanted between the skull and the brain surface. Data storage and signal telemetry takes place within the pectorally-implanted can that transmits signals wirelessly to an external handheld device that processes the data and transmits visual and audible signals to the patient. The external pager-like receiver displays a blue light when there is a low likelihood of seizures, white indicates medium susceptibility, and red alerts to a high likelihood of impending seizure.
Results of the system on 11 patients were published in the Lancet an a paper entitled “Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study.” From the paper:
“Methods
We enrolled patients at three centres in Melbourne, Australia, between March 24, 2010, and June 21, 2011. Eligible patients had between two and 12 disabling partial-onset seizures per month, a lateralised epileptogenic zone, and no history of psychogenic seizures. After devices were surgically implanted, patients entered a data collection phase, during which an algorithm for identification of periods of high, moderate, and low seizure likelihood was established. If the algorithm met performance criteria (ie, sensitivity of high-likelihood warnings greater than 65% and performance better than expected through chance prediction of randomly occurring events), patients then entered an advisory phase and received information about seizure likelihood. The primary endpoint was the number of device-related adverse events at 4 months after implantation. Our secondary endpoints were algorithm performance at the end of the data collection phase, clinical effectiveness (measures of anxiety, depression, seizure severity, and quality of life) 4 months after iniation of the advisory phase, and longer-term adverse events. This trial is registered with ClinicalTrials.gov, number NCT01043406.
Findings
We implanted 15 patients with the advisory system. 11 device-related adverse events were noted within four months of implantation, two of which were serious (device migration, seroma); an additional two serious adverse events occurred during the first year after implantation (device-related infection, device site reaction), but were resolved without further complication. The device met enabling criteria in 11 patients upon completion of the data collection phase, with high likelihood performance estimate sensitivities ranging from 65% to 100%. Three patients’ algorithms did not meet performance criteria and one patient required device removal because of an adverse event before sufficient training data were acquired. We detected no significant changes in clinical effectiveness measures between baseline and 4 months after implantation.
Interpretation
This study showed that intracranial electroencephalographic monitoring is feasible in ambulatory patients with drug-resistant epilepsy. If these findings are replicated in larger, longer studies, accurate definition of preictal electrical activity might improve understanding of seizure generation and eventually lead to new management strategies.”