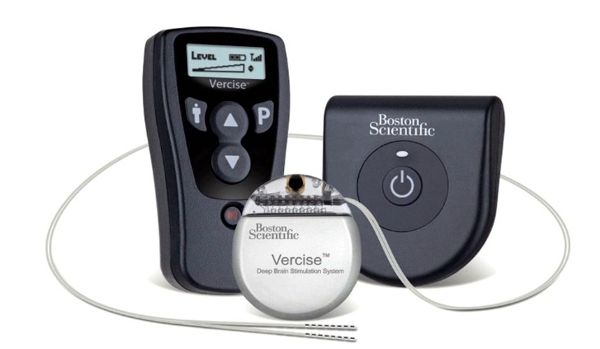
Boston Scientific announced today the first implantation of its Vercise™ DBS System as part of the INTREPID clinical trial. INTREPID is a prospective, multi-center, double-blinded, randomized, controlled study to evaluate the safety and effectiveness of motor function and overall quality of life in patients with the Vercise DBS System for the treatment of Parkinson’s disease. According to the press release:
The Vercise DBS System is a neurostimulation device that delivers electrical signals to specific areas within the brain through individual lead contacts. It is the only system that allows physicians to adjust the current flow in fine increments based on patient needs.
The first patient was implanted at Cedars-Sinai in Los Angeles by a team led by Michele Tagliati, M.D., director, Movement Disorders Program, and Adam Mamelak, M.D., FACS, FAANS, director, Functional Neurosurgery.
“The Vercise DBS System offers the promise to customize stimulation control in ways never before possible,” said Dr. Tagliati.
“The indications for deep brain stimulation are rapidly expanding, while innovations in the devices used for this therapy have been slower,” said Philip A. Starr, M.D., PhD, co-principal director, Functional Neurosurgery Program, University of California, San Francisco and co-principal investigator of the trial. “The launch of this trial heralds advanced innovation in device development which is greatly needed.”
“The Vercise DBS System has the ability to deliver stimulation in a precise manner due to multiple independent current control which represents a significant opportunity for DBS therapy in the treatment of Parkinson’s disease,” said Jerry Vitek, M.D., professor and chair, Department of Neurology, University of Minnesota and co-principal investigator of the trial. “The technology offered with this system is designed to provide a flexible programming platform that is expected to improve our patients’ quality of life.”
The Vercise DBS System has both CE Mark and TGA (Australia Therapeutic Goods Administration) approval and is available for sale in Europe, Israel and Australia. In the U.S., the Vercise DBS System is investigational and not available for use or sale.
Update 6/18/2013:
NATICK, Mass., June 18, 2013 /PRNewswire/ — Patients with Parkinson’s disease using the Boston Scientific Corporation (NYSE: BSX) Vercise™ DBS (deep brain stimulation) System showed a significant improvement in motor scores according to interim data from the VANTAGE DBS study. Data from the six month follow-up of up to 40 participants enrolled in the VANTAGE trial were presented at the annual International Congress of Parkinson’s Disease and Movement Disorders in Sydney, Australia by Prof. Dr. Lars Timmermann, of University Hospital in Koln, Germany.
The Vercise DBS System incorporates multiple independent current control, which is designed to selectively stimulate targeted areas in the brain, providing physicians with fine control of stimulation.
Preliminary analysis of the VANTAGE study displays approximately 60 percent mean improvement in motor function at six months post implant, as assessed by UPDRS III1 when compared to baseline. The Boston Scientific sponsored study was designed to document patient outcomes. These include effectiveness, safety, and health economic data derived from bilateral stimulation of the subthalamic nucleus (STN) in the brain using the implantable Vercise DBS System for the treatment of levodopa-responsive, moderate to severe idiopathic Parkinson’s disease. Forty participants with Parkinson’s disease were implanted bilaterally at six European centers.
“We are pleased to see such a significant improvement in motor function,” said Prof. Dr. Francois Alesch, professor for Stereotactic and Functional Neurosurgery at Medical University, Vienna, Austria and neurosurgical principal investigator of the trial. “I believe this unique technology, with its multiple current sources, may provide us with a more adaptable form of DBS therapy. I was very pleased with the simple recharging system. All of my patients were able to recharge successfully.”
Highlights of the VANTAGE study interim data include:
All 40 participants underwent successful implantation of the Vercise DBS System.
The Vercise DBS System demonstrated a significant improvement in motor function (p<0.0001), as assessed by UPDRS III (approximately 60 percent mean improvement) at six (6) months post first lead implant as compared with baseline.
Preliminary analysis suggests the Vercise DBS System improved participants’ ON time, as assessed by at-home motor diaries, activities of daily living and quality of life at six months.
The charging of the Vercise DBS System was well tolerated by all participants.