
Image Credit: Axonics
Axonics Modulation Technologies is an Irvine, CA company founded in 2013 that developed a novel implantable sacral nerve stimulator to treat patients with urinary and bowel dysfunction disorders.
The Axonics r-SNM system is a miniaturized rechargeable implantable stimulator with a longevity of at least 15 years. The system also features a charging system designed for quick charge times and a user-friendly remote control and programming interface.
The r-SNM device won CE Mark approval in June 2016, and is currently pursuing FDA approval using its “literature-based PMA submission”. This type of submission relies on supporting literature that is “sufficient, detailed, objective, and directly applicable to the subject device.” Axonics is quoting the Medtronic InterStim II device, which is the only U.S.-approved sacral neurostimulator. Axonics also provided to FDA one-year post-market data from its 51-patient European Relax-OAB study.
In February 2019 Axonics announced that its IDE pivotal clinical study demonstrated that patients implanted with the r-SNM system experienced clinically meaningful and statistically significant improvements in urinary urgency incontinence (UUI) symptoms and quality of life, met all secondary endpoints, and no serious device-related adverse events were reported.
Axonics had an Initial Public Offering in November 2018 which yielded $138M.
Axonics website: http://www.axonicsmodulation.com/
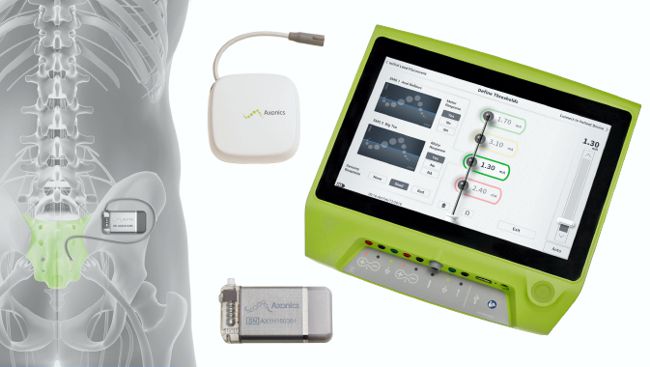
Image Credit: Axonics
