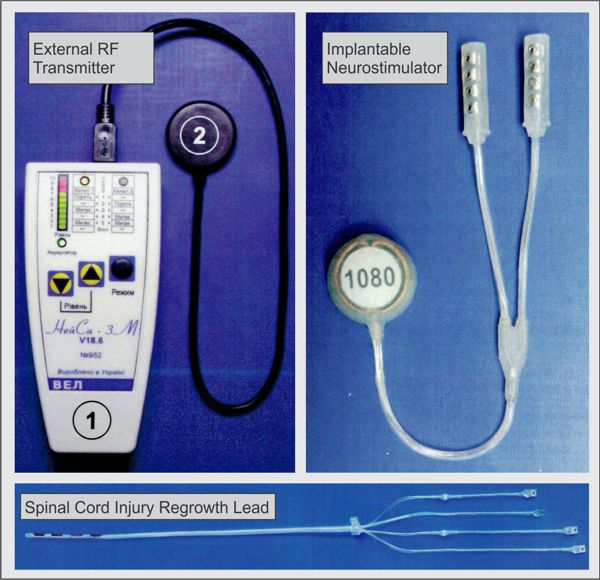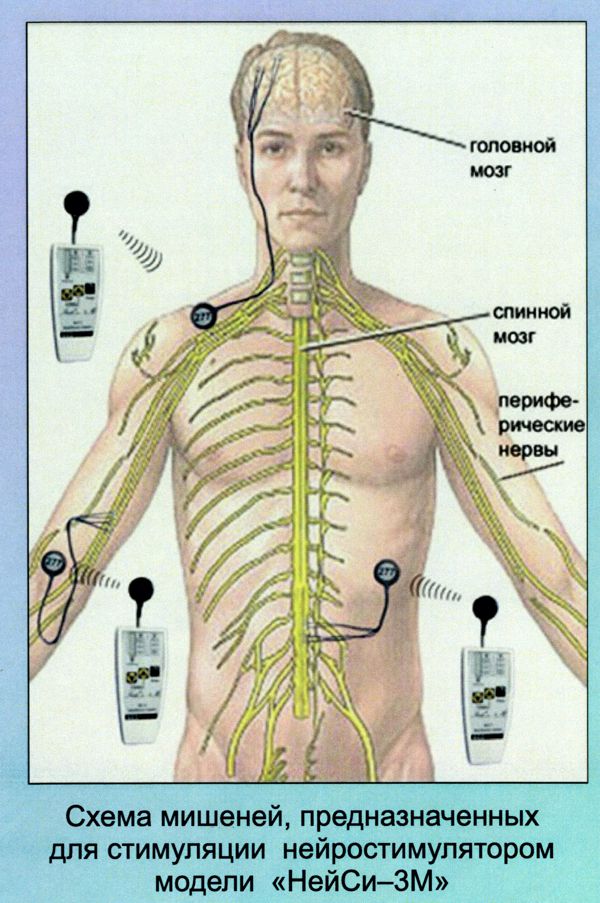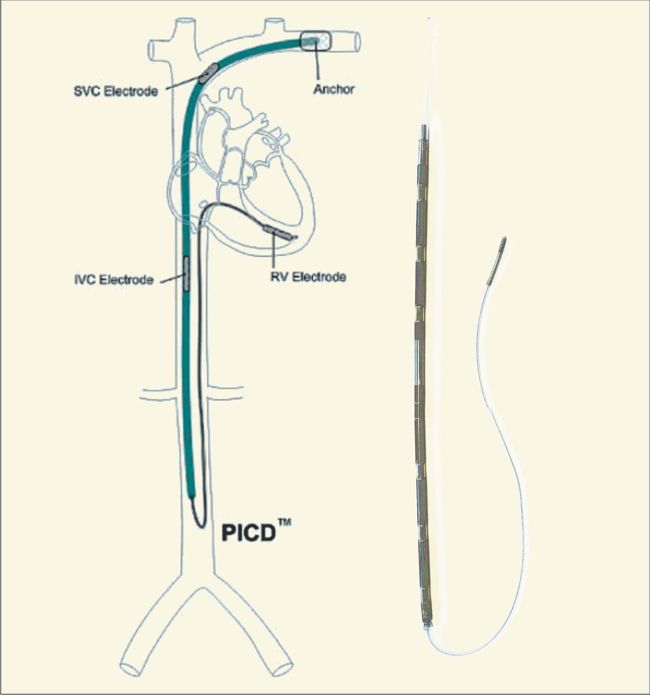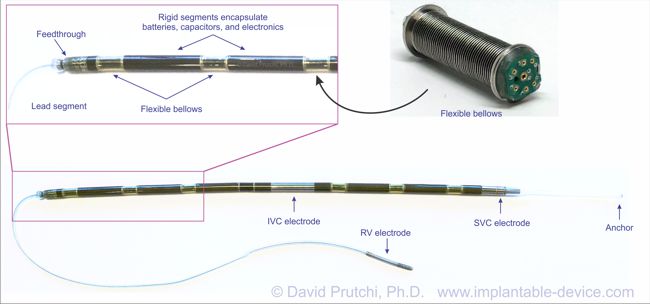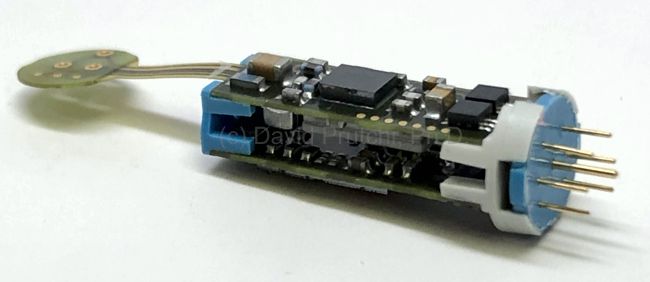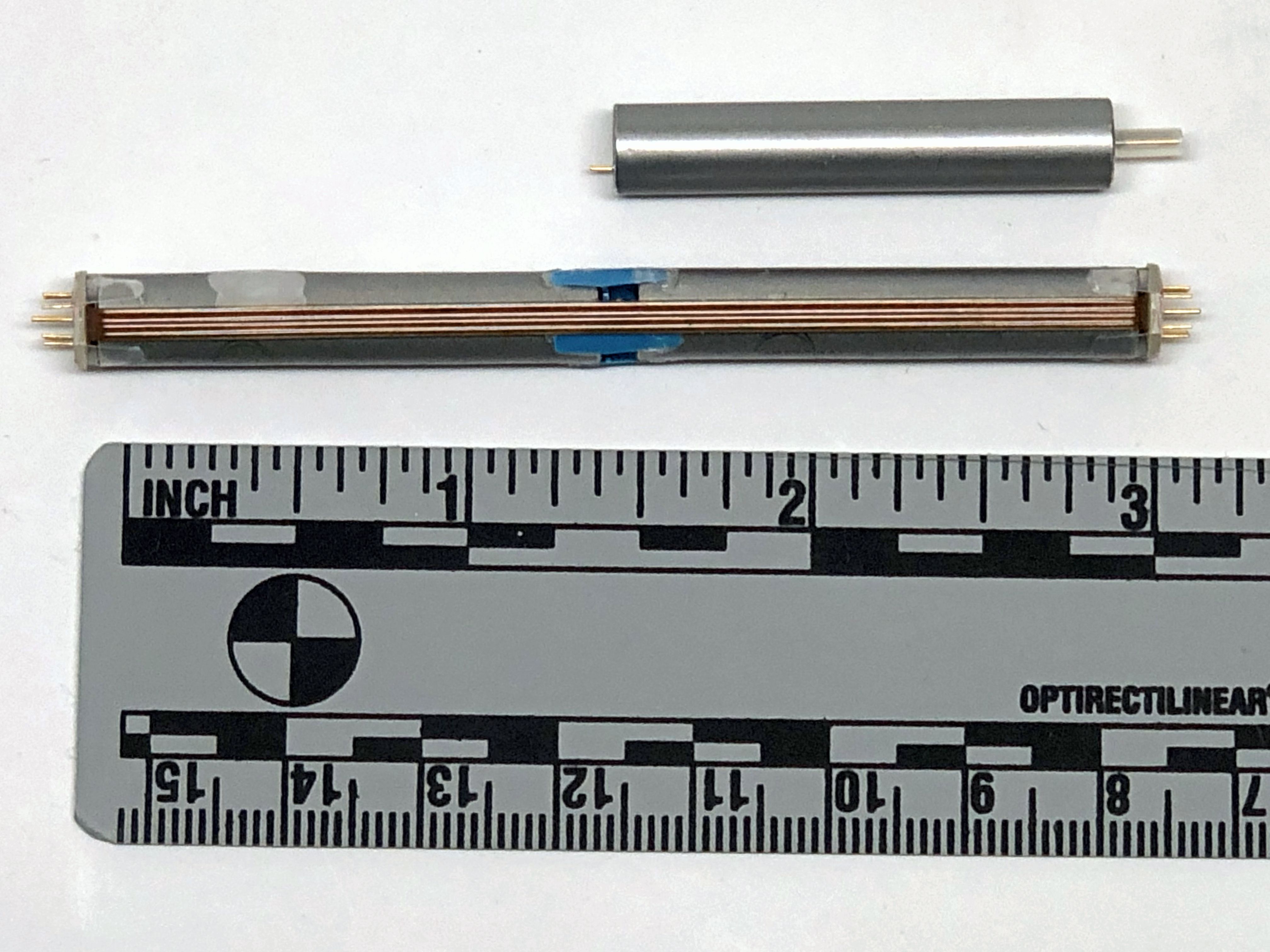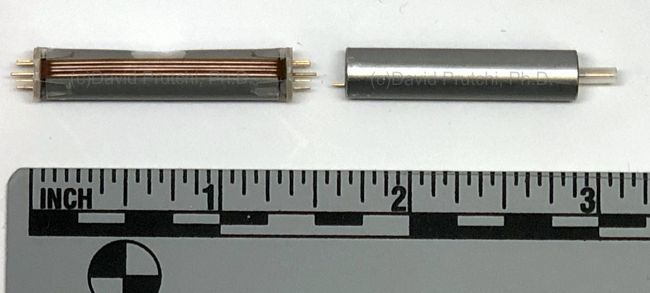
Image Credit: Medtronic
Things have gone well for Boston Scientific with its subcutaneous implantable cardioverter defibrillator (S-ICD). These devices have no transvenous leads, and as such provide a solution for patients at risk for SCD when implantation of a traditional ICD with transvenous leads is not possible or desired.
S-ICDs have their disadvantages though – current models are larger in size because of the high energy they must deliver to defibrillate from a subcutaneous lead (as a reference, Boston Scientific’s EMBLEM S-ICD delivers 80J shocks), and do not provide antitachycardia pacing (ATP) or bradycardia pacing support, except for limited post-shock pacing.
Medtronic is proposing a different approach to Boston Scientific’s. They call it the Extravascular Implantable Cardioverter Defibrillator (EV ICD), which uses a lead placed under the sternum, which gets the electrodes much closer to the heart than Boston Scientific’s subcutaneous lead.




 I have led the development of devices to deliver CCM™ therapy from the very beginning, so it is with great pleasure and pride that I share with you the exciting news that Impulse Dynamics just received approval from the United States Food and Drug Administration (FDA) for our Optimizer® Smart System for heart failure patients! An official FDA announcement was made through the publication of a press release that you can find here:
I have led the development of devices to deliver CCM™ therapy from the very beginning, so it is with great pleasure and pride that I share with you the exciting news that Impulse Dynamics just received approval from the United States Food and Drug Administration (FDA) for our Optimizer® Smart System for heart failure patients! An official FDA announcement was made through the publication of a press release that you can find here: