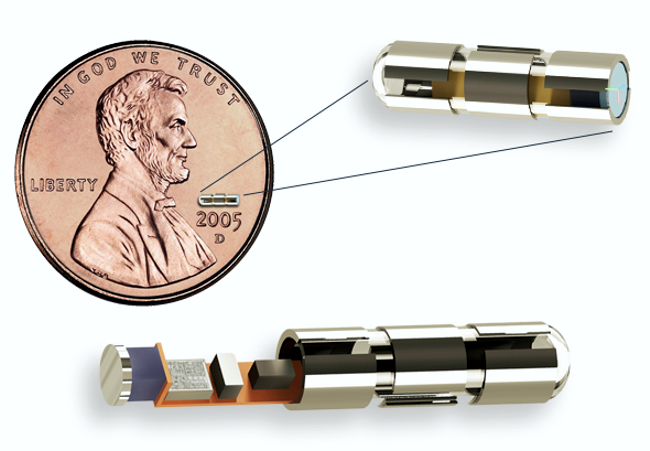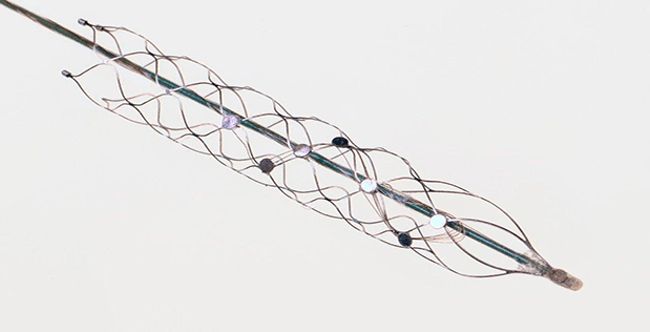
Image Credit: Synchron
Synchron Inc. announced today the first successful clinical implantation of the Stentrode®, a minimally-invasive neural interface technology, a component of the Synchron Brain-Computer Interface. This is the first clinical feasibility trial evaluating this technology for its potential to restore communication in people with severe paralysis.