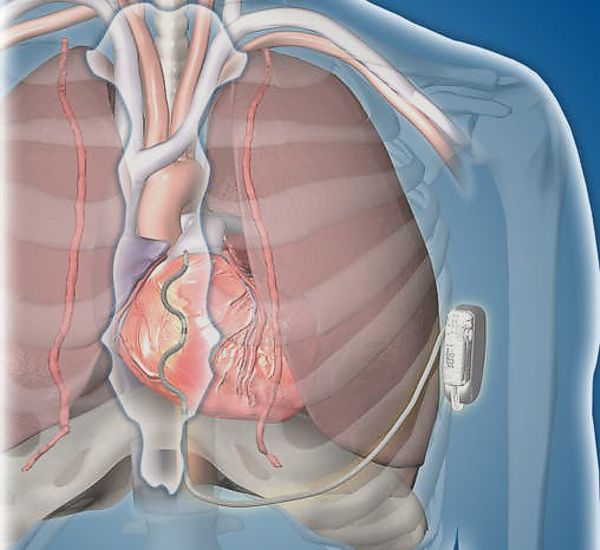
Image Credit: Medtronic
Medtronic announced today that it started a worldwide pivotal study evaluating its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system. The EV ICD system is designed to deliver lifesaving defibrillation and pacing therapy via a device the same size as traditional, transvenous ICDs, but with an extravascular lead placed outside the heart and veins.
According to the press release:
“The EV ICD pivotal study is a prospective, multicenter, single-arm, non-randomized, pre-market clinical study that will assess the Medtronic EV ICD system in up to 400 patients at up to 60 sites in North America, Europe, the Middle East, Asia, Australia and New Zealand.
The study’s primary effectiveness endpoint is defibrillation testing success rate at implant. The primary safety objective is freedom from major system and/or procedural complications at six months after implant. Patients will be assessed at two weeks, three months, six months, and every six months thereafter.”
