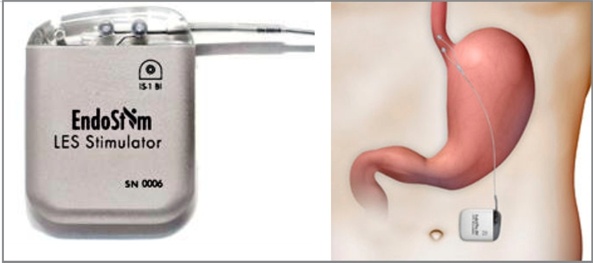
EndoStim was a medical device company based in Dallas, Texas, and Nijmegen, The Netherlands, developing and commercializing a treatment for gastroesophageal reflux disease (GERD).
On October 15, 2019 EndoStim terminated its Clinical Investigation of the EndoStim® Lower Esophageal Sphincter (LES) Stimulation System due to lack of funding. According to a letter sent by EndoStim to its investigators, clinical and technical support will no longer be available for the devices after January 14, 2020
EndoStim terminated operations and entered into an Assignment for Benefit of Creditors under Delaware law, which is a state law liquidation procedure similar to a Chapter 7 bankruptcy.


