Image Credit: Zurich MedTech
The FDA announced the acceptance of Zurich MedTech’s Sim4Life IMAnalytics and the field exposure libraries MRIxVIP1.5T and MRIxViP3.0T from the IT’IS Foundation as a Medical Device Development tool (MDDT). This is the first FDA-approved computational modeling Medical Device Development Tool.
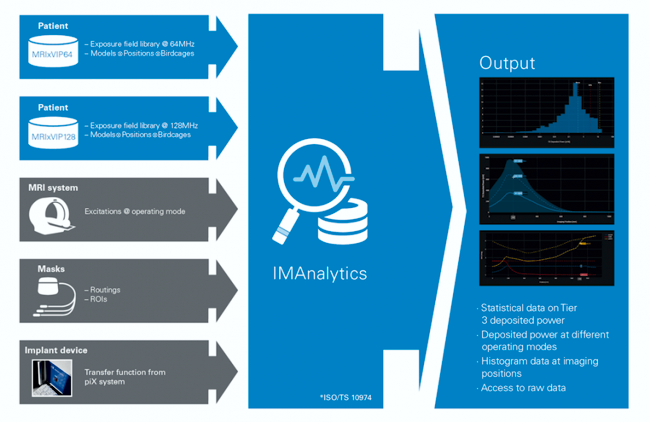
IMAnalytics is a software platform for the safety evaluation of implantable devices in the Magnetic Resonance (MR) environment. It characterizes RF-induced heating at the distal electrodes of implantable devices, using a variant of the Tier 3 approach as defined in ISO/TS 10974. It is tailored for elongated lead structures by making use of the transfer function method described in Annex K of the same technical specification.
From the press release:
“According to Edward Margerrison, Ph.D., Director, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health (CDRH), FDA, “IMAnalytics with MRIxViP1.5T/3.0T and BCLib Toolset may be used in the premarket submissions of AIMDs to obtain the statistical distribution of the in vivo deposited power and/or induced terminal voltage to support MR Conditional labeling of these medical devices for 1.5T or 3T MR scanners, according to the Tier 3 approach defined in ISO/TS 10974:2018”. In other words, applying a qualified MDDT tool as non-clinical assessment model eliminates much of the risk and uncertainty manufacturers often experience in product development”
