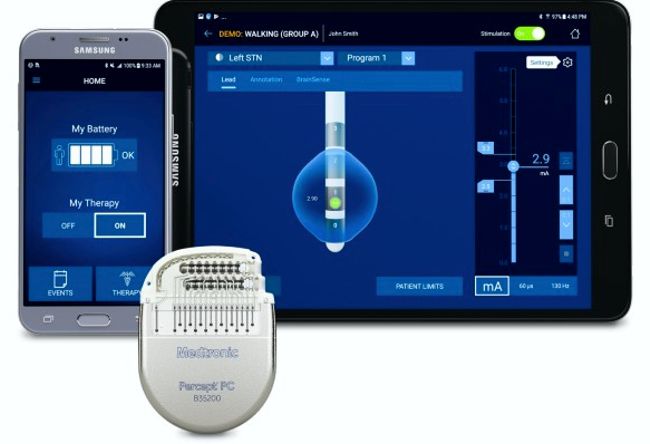
Image Credit: Medtronic
Medtronic announced that it received the CE Mark for Percept™ PC neurostimulator. The Percept™ PC neurostimulator; is the only Deep Brain Stimulation (DBS) system to be launched in the European Union (EU) with BrainSense™ technology that can sense and record brain signals while delivering therapy to patients with neurologic disorders, such as Parkinson’s disease.
According to the press-release:
“BrainSense technology enables physicians to track patient-specific brain signals and correlate these with patient-recorded events, such as symptoms or side-effects associated with their disease or the medications to treat it. This enables more personalized, data-driven neurostimulation treatment. The Percept PC neurostimulator is approved in the EU for the treatment of symptoms associated with Parkinson’s disease (PD), essential tremor, primary dystonia as well as epilepsy and obsessive-compulsive disorder (OCD). It is currently under review by the U.S. Food and Drug Administration.
“DBS is proven to significantly improve motor function in people with Parkinson’s disease compared to standard medication alone – but with currently-available systems, physicians need to make therapeutic decisions mostly based on clinical assessments and patient-reported information,” said Professor Andrea Kühn, head of Movement Disorders and Neuromodulation, Charité University Hospital, Berlin. “Percept PC with BrainSense technology is a game changer. Patients and their care teams will have objective patient-specific brain signal data – including data recorded outside the clinic in patients’ everyday lives. With this technology, doctors could tailor therapy more precisely to the individual needs of each patient based on data from neuronal activity.” “
