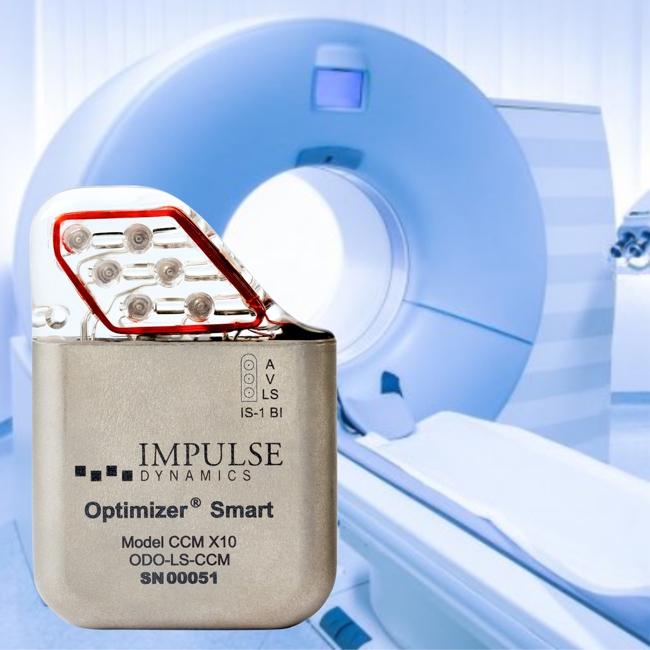
Impulse Dynamics – the company for which I am CTO and Executive VP – announced today that it received European approval for labeling the OPTIMIZER Smart IPG as MR-Compliant for full-body MRI scans utilizing 1.5 Tesla scanners:
GLOBAL REGULATORY ORGANIZATION CERTIFIES OPTIMIZER SMART DEVICE
as compliant for WHOLE-BODY MRI SCANS IN THE EUImpulse Dynamics Receives Medical Device Approval for European Union to Safely Administer MRI Scans for Heart Failure Patients with Implanted Optimizer Smart Devices
MARLTON, N.J., February 16, 2021 — Impulse Dynamics, an international medical device company dedicated to improving the lives of people with heart failure (HF), today announced that DEKRA Certification B.V. (DEKRA), a global regulatory organization, approved labeling for the Optimizer® Smart device for conditional full-body MRI scans utilizing 1.5 Tesla scanners in the EU. DEKRA, known for ensuring the safe use of medical devices in global markets, has given HF patients with the Optimizer Smart device implant the opportunity to receive whole-body magnetic resonance imaging required for the diagnosis of anatomical and physiological processes.
The commercial significance of this accomplishment affords not only HF patients with peace of mind but healthcare practitioners with unhindered access to perform full-body MRI scans. Additionally, the Optimizer’s safe use approval opens the door to extensive use with other MRI-conditional devices. “The MRI full-body certification by DEKRA is a significant step for Impulse Dynamics. This gives patients and physicians the assurance that important diagnostic examinations can be performed for the benefit of patients, independent of the CCM therapy,” Jens Kalender, Vice President of Commercial DACH for Impulse Dynamics, said today.
Additionally, as noted by Deborah Morley, Ph.D., Vice President of Regulatory Affairs and Quality Assurance, “Attainment of whole-body MRI-conditional CE labeling is a major milestone for Impulse Dynamics made possible through positive cooperation with the notified body, DEKRA, and our top-notch engineering team. We look forward to many more such achievements in the near future, advancing CCM therapy to provide hope for heart failure patients worldwide.”
