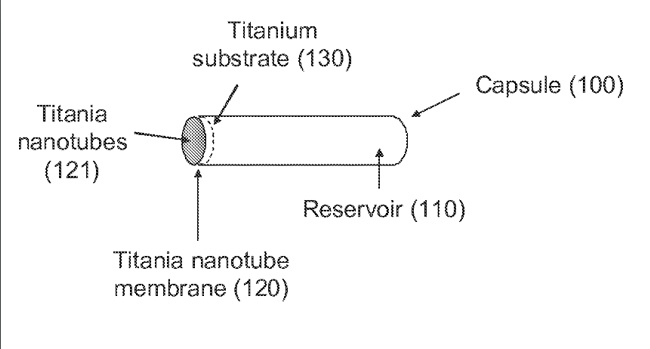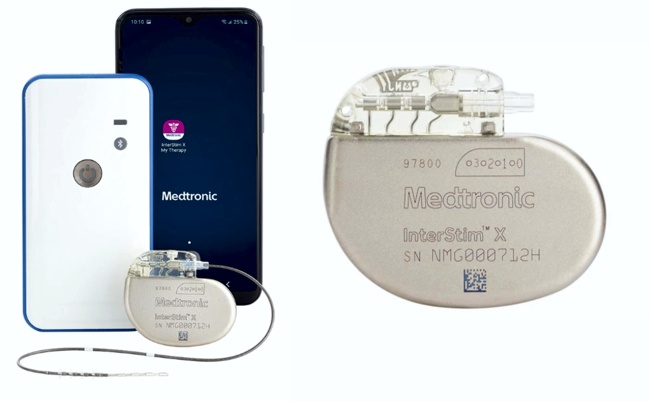
Image Credit: Medtronic
Medtronic announced that it received approval from the U.S. Food and Drug Administration (FDA) for its InterStim X™ sacral neuromodulation system for the treatment of overactive bladder (OAB), chronic fecal incontinence (FI), and non-obstructive urinary retention.
According to the announcement, the InterStim X device features a proprietary 5th generation battery chemistry that offers more than 10 years of battery life without the need to recharge, providing patients with more freedom and less maintenance. With low energy settings, the device may last up to 15 years.