Image Credit: Medtronic
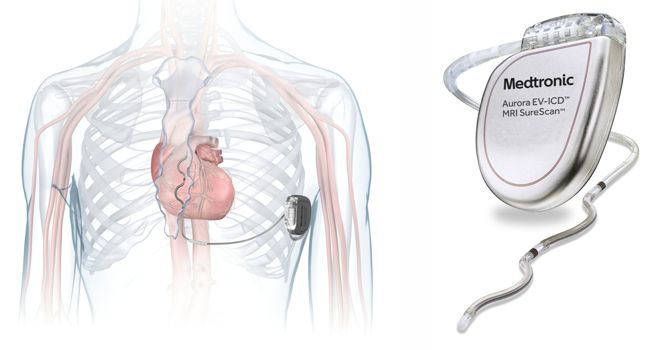
Medtronic announced today that it received FDA approval for its Aurora EV-ICD™ MRI SureScan™ Extravascular Implantable Cardioverter-Defibrillator and Epsila EV™ MRI SureScan™ defibrillation lead.
According to the press release:
“The Aurora EV-ICD system is the first-of-its-kind to provide the life-saving benefits of traditional, transvenous ICDs with a lead (thin wire) placed under the breastbone, outside of the heart and veins. The Aurora EV-ICD delivers lifesaving defibrillation, anti-tachycardia pacing (ATP), and back-up (pause-prevention) pacing therapies via a device similar in size, shape, and longevity to traditional, transvenous ICDs.”
“The Aurora EV-ICD system includes features available in Medtronic transvenous ICDs, and offers additional advantages that are not available with the subcutaneous ICD including:
-
- Anti-tachycardia Pacing (ATP), to terminate ventricular arrhythmias (rapid and/or chaotic activity of the heart that can lead to SCA) using low-energy pacing pulses, potentially avoiding a defibrillation shock.
- Pause Prevention Pacing, to provide back-up pacing for brief, intermittent, heartbeat pauses.
- 40 Joule Defibrillation Energy, to deliver life-saving shocks in a device the size of transvenous ICDs (33 cc)
- Medtronic exclusive PhysioCurve™ design, to increase patient comfort and implant acceptance.
- 11.7-year projected longevity, to reduce device replacement procedures during a patient’s lifetime.”
