Image Credit: Flowonix Medical
Yesterday I was chatting with a friend regarding the medical device industry in NJ, and realized that I never posted a blog on Flowonix based in Mount Olive, NJ.
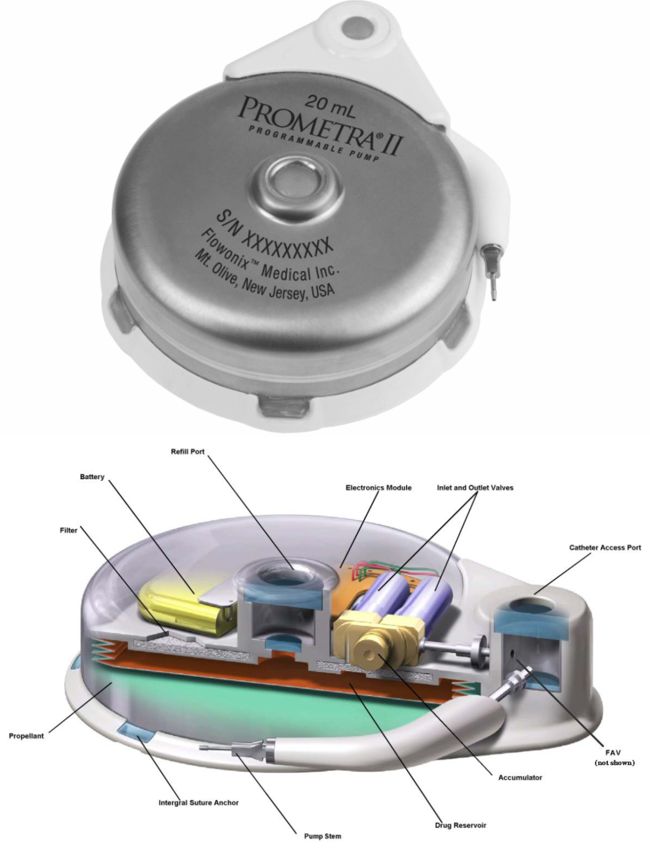
Flowonix was founded in 2005 (then called Medasys). It received approval to conduct its first clinical trial in 2007 on the Prometra programmable implantable pump. The company received approval by the FDA to market the Prometra in 2012 to deliver Infumorph.
The Prometra II Programmable Pump has a precisely-controlled flow controller system, which dispenses infusate into the intrathecal space through an implanted intrathecal catheter. To help increase safety, the Prometra II Pump incorporates a safety valve (flow-activated valve or FAV) that will shut off drug flow to the patient in the event a high flow rate occurs, such as during an MRI. All functions of the system (e.g., dosing) are controlled externally using a hand-held, battery-operated programmer.
Unlike competitor’s motor-driven pumps, the Prometra II Pump utilizes a pressure-driven, valve-gated delivery mechanism to deliver boluses of medication into the intrathecal space. This innovative delivery mechanism provides broad spinal cord coverage rapidly and enables novel programming modes of intermittent flow followed by periods of no flow, unique to the Prometra device. The Prometra device also features a 10+ year battery life, which can significantly reduce the number of future surgical procedures patients will need to replace expiring pumps.
The Prometra II Pump contains a metal bellows drug reservoir. There are two models, with capacities of 20 and 40 ml (introduced in 2019). The reservoir propellant is stored within the rigid housing surrounding the bellows and provides the driving pressure for the pump. The driving pressure on the reservoir forces the infusate through an outlet filter, and into an electronically controlled flow metering valve-accumulator subsystem. The Infumorph passes from the flow metering subsystem, into the catheter access port then into the catheter for delivery to the intrathecal space.
In 2020 FDA authorized the Prometra II for use with intrathecal baclofen for the treatment of severe spasticity, a condition which affects more than 12 million people worldwide, with common conditions such as cerebral palsy (CP), multiple sclerosis (MS), traumatic brain injury (TBI), spinal cord injury (SCI) and stroke comprising the majority of that population.
