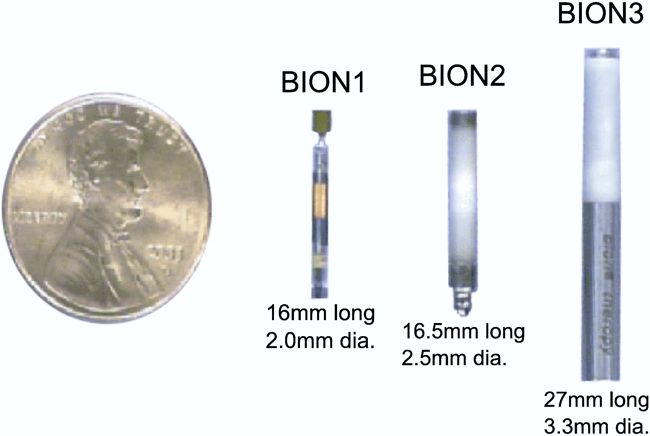
First-generation RF-powered BION (RFB1) microstimulator. Image Credit: Alfred Mann Institute for Biomedical Engineering
In 1991, Dr. Gerald Loeb, at the time a Professor of Physiology and Biomedical Engineering at Queen’s University (Kingston, Canada), first proposed a miniature, injectable, RF-powered device for the stimulation of tissue or motor neurons. The BION® device was developed based on this concept as a joint project between Queens University (Kingston, ON, Canada), IIT (Chicago, IL), and the Alfred E. Mann Foundation (Valencia, CA) with funding from the NIH Neural Prosthesis Program. The RF BION 1 (RFB1) was then manufactured by the Alfred Mann Institute for Biomedical Engineering at USC.
The RFB1 had a small circuit embedded within a glass enclosure containing a ferrite-wound coil to receive RF energy and stimulation commands, and an ASIC for control and stimulation switching. A cylindrical tantalum oxide electrode served as both the energy-storage capacitor as well as the stimulation electrode (cathode). An iridium plate served as the return (anode) electrode. Phase balancing was achieved during the recharge of the tantalum oxide electrode.
The RFB1 could stimulate tissue with pulses with a maximum duration of 512ms and 30mA. Using short pulses, the device could produce pulse trains at rates of around 500Hz. Each RFB1 was assigned a unique 8-bit address, so a single external controller could independently power and control up to 255 implanted stimulators.
A second-generation RF-powered BION (RFB2) was developed in the early 2000s by the Alfred E. Mann Foundation. It used the same basic circuitry as the RFB1, but had an internal stimulation capacitor. It enclosed the circuitry in a hermetic ceramic case, and used platinum-iridium electrodes. The device also had a small suture eyelet to facilitate positioning.
Simultaneously, a battery-powered BION (BION 3) was developed by Advanced Bionics, which at the time was one of Al Mann’s companies. The device was capable of producing pulses with a maximum current of 112mA and a frequency of over 1,000Hz. The device included memory to store stimulation parameters programmed by a physician through an external programmer. Power came from a 10mWh lithium-ion cell manufactured by Quallion (also an Al Mann company at that time, now owned by Enersys) that could be recharged transcutaneously by the patient at home. Patients were provided with a Remote Control to allow them to turn stimulation ON or OFF, change stimulation strength, and check the charge level of the device’s rechargeable battery.
The battery-powered BION had received CE Mark approval for its use to treat urinary urge incontinence and was in the midst of a clinical trial to receive FDA approval, when in 2004 Boston Scientific acquired Advanced Bionics.
Prior to the acquisition, Advanced Bionics had demonstrated a multi-channel BION which was being designed, among other things, for the treatment of migraine headaches. However, Boston Scientific allowed BION devices languish after it estimated that it would cost an additional $35M to complete the development for that application.
Boston Scientific’s halting the production and further development of the BION left an unfulfilled gap in the urinary incontinence space. Dr. Loeb, now at USC, thus developed the NuStim® – a similar RF-powered stimulator that bypassed the BION IP – by partnering with General Stim – a Chinese company with a development office in Los Angeles.
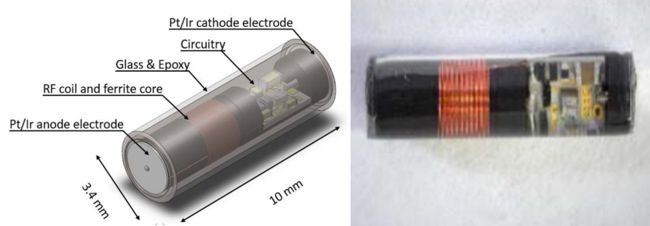
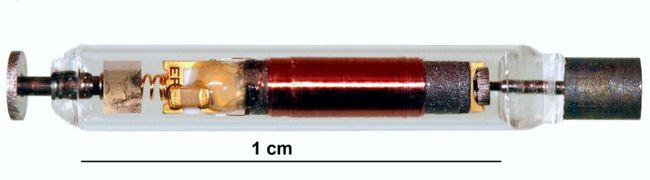
A 2017 paper in IEEE Trans. Neural Systems and Rehabilitation Engineering describes the NuStim and its construction in detail. In essence, the device’s circuit is constructed from off-the-shelf components encapsulated in a borosilicate glass tube filled with epoxy. Pt/Ir electrodes at both ends of the glass tube complete the stimulator. The RF transmitter is part of a seat cushion, and stimulation parameters are set from a tablet-based app.
I was recently contacted by someone who was interested in using a NuStim for a veterinary application. Looking into it, it seems that NuStim production was abandoned by General Stim, which is owned by the Chinese cochlear implant manufacturer Nurotron.
On the meantime, other players have appeared with microstimulators for the treatment of urinary incontinence, including Nuespera, Axonix, Valencia Technologies, and BlueWind. Externally-powered microstimulators for other functions include the eAxon, Motif, Nalu, etc.