
Image Credit: EPFL. This implant measures about 14mm and comprises five sensors, a coil for wireless power as well a miniaturized electronics for radio communication
From Wired:
Written by Liat Clark
Edited by Nate Lanxon
“A multidisciplinary Swiss team has developed a tiny, implantable device that instantly analyses the blood before wirelessly sending the data to a doctor.
The device can be used for monitoring general health, but the team also sees immediate applications in monitoring the efficacy of treatments such as chemotherapy in order to tailor drug delivery to a patient’s unique needs.
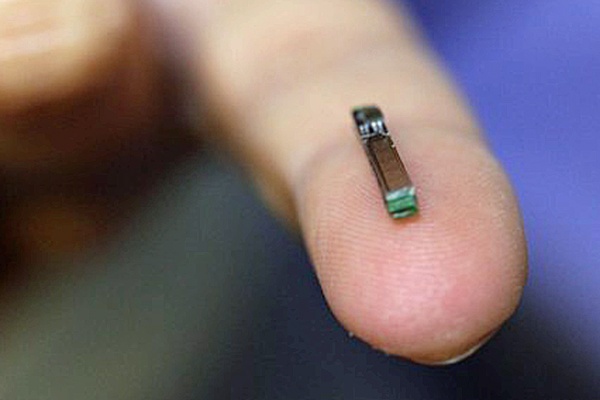 Giovanni de Micheli and Sandro Carrara of École Polytechnique Fédérale de Lausanne (EPFL) invented the device, drawing on expertise from the multidisciplinary school to develop the 14mm-long chip, which is fitted with five sensors and a radio transmitter and is powered via inductive coupling with a battery patch worn outside the body delivering a tenth of a watt in energy. The flexible patch is also Bluetooth-equipped to transfer the data picked up by the chip’s radio signals.
Giovanni de Micheli and Sandro Carrara of École Polytechnique Fédérale de Lausanne (EPFL) invented the device, drawing on expertise from the multidisciplinary school to develop the 14mm-long chip, which is fitted with five sensors and a radio transmitter and is powered via inductive coupling with a battery patch worn outside the body delivering a tenth of a watt in energy. The flexible patch is also Bluetooth-equipped to transfer the data picked up by the chip’s radio signals.
It can be implanted in the interstitial tissue — the tissue between cell structures, already identified as a good location for testing for compounds in the blood — but Carrara tells Wired.co.uk it could one day be implanted in the arms and legs of athletes: “We could monitor them as we do nowadays with Formula One cars. We can even locate the chip in the arms or in the leg, places that are more under pressure during a match.”
The chip has already been used to accurately test for a whole series of molecules, such as lactate and glucose, with results deemed as reliable as traditional methods — the difference is they are instantaneous, delivering data to a doctor as incremental changes occur in the body.
Implantable diagnostic tools are not new. Research institutes have been working on biosensors that measure glucose for years to make the lives of diabetics easier. However, other products edging towards the marketplace tend to focus on solving one easily identifiable problem. de Micheli and Carrara are thinking big, with their chip able to be tailored to many different issues, using sensors loaded with different proteins to test for multiple diseases.
“It’s the first system in the world that’s so small but can target up to five different molecules,” Carrara tells Wired.co.uk, “that means you can follow several diseases.”
He and de Micheli plan on first applying for an application to test for inflammation of the gut in animals — because it releases so many different molecules — then another for research into its uses in anesthetics and intensive care, to monitor health post-surgery. Essentially, they hope to personalise medical care at every stage in the system.
“When you supply any drugs to a patient you have their immune system and metabolic system that, to some extent work against you,” says Carrara. “Each of us has our own immune and metabolic system. The point is even the anesthesiologist does not know how this particular patient will behave to drugs. So in order to have an online monitoring system that can immediately provide the feedback of how the compounds are going into the bloodstream is of extreme importance for the doctor to fine tune it.”
According to a study into post-surgery mortality rates by Queen Mary University of London, death from all causes is four percent across 28 European countries. Figures varied massively according to the country’s post-operative care, with a rate of 21.5 percent in Latvia and 1.2 percent in Iceland. It is a stark reminder that medical care must go beyond what we can see — incremental changes at the molecular level post-surgery could be indicators of organ failure, heart attacks — all manner of life-threatening events. As long as there’s a molecular indicator, the chip can provide a solution argue the EFPL team — troponin, for example, is released by the heart prior to a heart attacks, so if picked up early treatment could be preempted faster than ever before.
According to the NHS, coronary heart disease is the UK’s biggest killer, with 82,000 people dying as a result every year. If at-risk patients were fitted with the chip, that number could dramatically drop.
On the flipside, that kind of monitoring might require a semi-permanent sensor which, right now, is technologically out of reach if based on proteins.
“The longest experiment so far was one month in mice,” says Carrara. “We studied its biocompatibility and the tests were very good. We are not limited to electrical or mechanical signals in the body, but we are really targeting molecular signals. However for that we really need specific proteins that target specific molecules, and that’s is the soft part. Of course our body has been built to substitute our proteins almost on a daily basis. There has been a huge effort to elongate the lifetimes of the proteins, but it’s not scientifically feasible right now to have a full-life implanted biosensor that is based on enzymes that last longer.”
One industry partner that has shown interest in picking the project up (the team has had plenty of interest, but cannot divulge from whom) has suggested using an implant that can be injected with a syringe, and easily removed via the same pathway
“In this case the implant will be really easily injected, with no surgery required, so we can imagine if a chronic patient needs to be monitored for their whole life they could go to the doctor once a year to change the implant and have continuous monitoring.”
A year-long glucose sensor implant for diabetes has already successfully been tested elsewhere, but for most uses implants don’t need to be operational for so long. For instance, another area ripe for this kind of personalisation is chemotherapy.
There remains, in cancer care, an element of guesswork involved in administering chemotherapy. A cocktail of what is essentially poison is administered by an oncologist, who carefully studies the case before giving the best possible combination of drugs for the particular cancer targeted. However, how the patient’s body — and their body’s cancer — reacts to those drugs, is an unknown.
There are so many unknowns involved, doctors usually take blood work to assess white blood cell count and other indicators after it has been administered, and after several rounds. If it’s not the right combination for the particular patient, precious time has been wasted while the cancer possibly continues to grow.
Last year Toronto-based Rna Diagnostics announced it had developed a tool for testing how a breast cancer patient has responded after the first cycle of chemo, after discovering that the quality of Rna is a direct indicator of whether or not the drugs are in fact working. With EPFL’s chip, however, results could one day be instantaneous and detailed, reflecting exactly how the body is responding to the drugs so they can be tweaked accordingly.
“For chemotherapy it’s more than enough to do it for few hours, a few days max. A couple of days is more than enough for any personalisation for drug treatment. We’ve already tested four anti-cancer compounds and of course we are developing the technology to monitor these kinds of compounds online.”
The device has already been tested in mice, but human trials are not too distant, with the first ones slated to take place in three years time (the team is in talks with a Swiss hospital to start trials there) and the device brought to market in four years. The government has already invested around €5 million (£4.3 million) in the project, but once human trials get the go ahead the likelihood is industry will bring a lot more to the table and help ready it for manufacture.
Before then, the team will continue to improve upon battery power, focusing on keeping everything as lightweight as possible. But with proof of concept already attained, even exceeded, the possibilities are exciting.”
Click here for the press release
