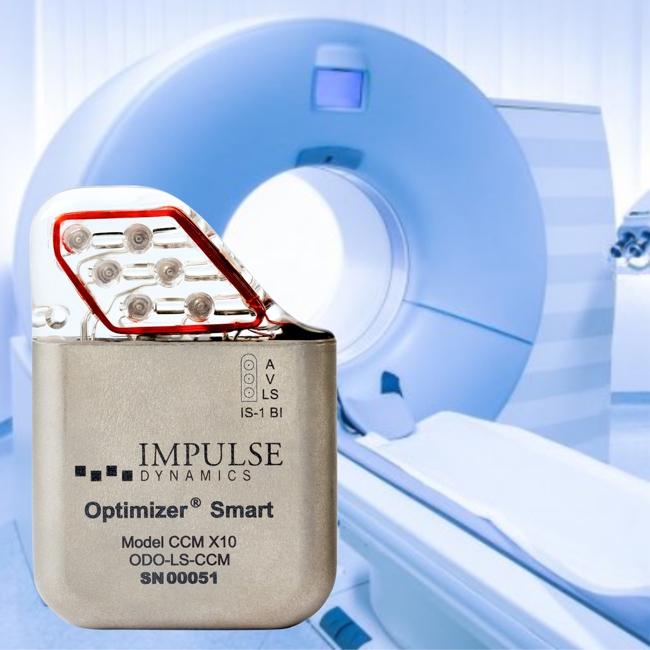
Impulse Dynamics – the company for which I am CTO and Executive VP – received MR-conditional approval (1.5 T head/limbs with peripheral coils) from both FDA and the European Union.
From the press release:
IMPULSE DYNAMICS ANNOUNCES FDA APPROVAL FOR MAGNETIC RESONANCE IMAGING FDA
Clears Potential Hurdle for Many Heart Failure Patients
MARLTON, N.J.–(BUSINESS WIRE)–Impulse Dynamics, a company dedicated to improving the lives of people with heart failure (HF), today announced the U.S. Food and Drug Administration (FDA) has approved the conditional use of Magnetic Resonance Imaging (MRI) for Optimizer® CCM® delivery systems. This approval represents a significant advance because the population that benefits most from cardiac contractility modulation therapy (patients with moderate to severe HF) often requires advanced diagnostic imaging procedures.
“It’s important that Impulse Dynamics acted quickly in a joint effort with the FDA to remove this potential barrier standing in the way of critical diagnostic procedures for a subset of patients with HF. This technology has received a lot of attention in our facility, and, likely, many of the patients who need and then receive CCM therapy will ultimately require MRI procedures,” said Dr. Robert W. W. Biederman, Director of Cardiovascular Imaging and Cardiac MRI at Allegheny General Hospital/Allegheny Health System in Pittsburgh and an author on the groundbreaking 2017 MagnaSafe trial published in NEJM. “Certainly, other imaging modalities are available, but MRI is the gold standard in terms of image quality, especially for neurovascular imaging. Typically, MRI is preferred by imaging specialists because it’s the safest alternative for most patients. Further, combined with the early successes in improving cardiac performance, now when combined with MRI compatibility, CCM adds to the armamentarium of the cardiologist and electrophysiologist in further optimizing FDA-approved medical therapies in 2021.”
The Optimizer device, similar in size to a pacemaker, is implanted during a minimally invasive procedure while the patient is under light sedation. The device provides CCM therapy, consisting of precisely timed electrical pulses delivered to the heart during the absolute refractory period of the beating cycle, just after the heart contracts. It has been used to treat over 5,000 patients and is currently available in the United States, Europe, China, Brazil, India, and over 40 other countries worldwide. This U.S. approval complements previous MR-conditional approvals in geographies outside of the United States. Providers are encouraged to reference the package insert related to MRI safety for specific details regarding implant conditions and scanning procedures in their respective geographies.
“We appreciate the FDA’s open and willing collaboration on this submission and the approval process,” said Dr. David Prutchi, Executive Vice President and Chief Technology Officer at Impulse Dynamics. “Our goal was to prove that patients implanted with CCM delivery devices could safely undergo MRI scans of the head and limbs using local RF transmit-receive coils. With that objective met, we will continue to press forward in our efforts to advance CCM therapy with additional clinically meaningful improvements.”
CCM therapy is the first approach designed to optimize heart contraction, allowing more oxygen-rich blood to reach the body. This breakthrough technology can improve the quality of life for patients who are no longer adequately responding to medications to manage their symptoms or slow their condition progression.
Impulse Dynamics website: www.impulse-dynamics.com
