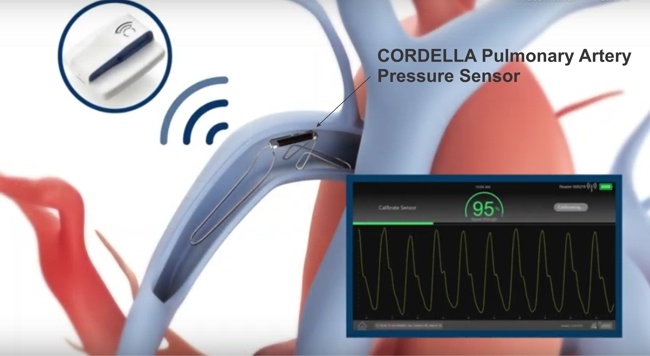
Endotronix, announced the submission of a Premarket Approval (PMA) application for its Cordella™ Pulmonary Artery (PA) Sensor System to the FDA.
According to the announcement:
“Cordella is an innovative HF patient management platform that delivers proactive PA pressure data and non-invasive vital health data for comprehensive heart failure management at home. Its user-friendly devices securely transmit daily health information to the managing clinician, supporting optimal dosing of guideline-directed medical therapy (GDMT) to reduce congestion and engaging patients with trended health data to enable healthy lifestyle choices.”
In April 2023 Endotronix completed the enrollment of its PROACTIVE-HF pivotal study. Primary endpoint data from the trial is expected to support the PMA Application.
