 InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
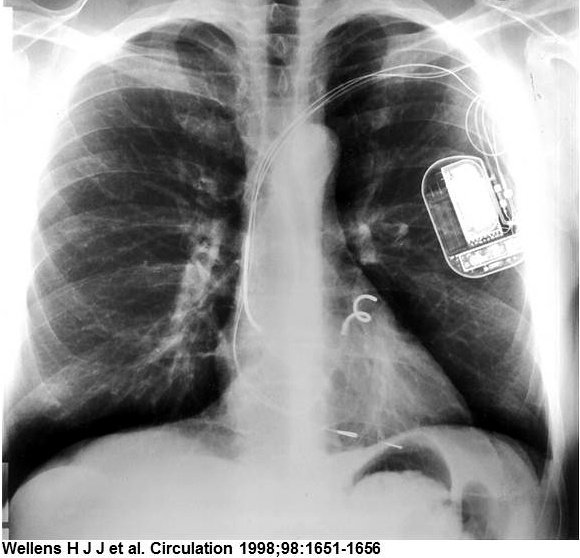
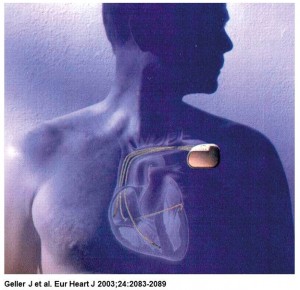
The implantable atrioverter system consisted of an implantable atrial defibrillator (model 3000 or 3020) connected to right atrial (perimeter right atrial model 7205) and coronary sinus (perimeter coronary sinus model 7109) defibrillation leads and a bipolar endocardial ventricular pacing lead, a programmer, and a defibrillation systems analyzer. The device had a volume of 53cc and weighed 79 g (model 3000) or 82 g (model 3020).
The device detected AF and delivers R-wave synchronous defibrillation shocks to convert AF to sinus rhythm. It was also able to pace the ventricle after shock delivery in case of bradycardia. Shocks could be delivered at a selected voltage up to 300V. The model 3000 defibrillator had an 80µF capacitor and could deliver a maximal shock of 3J with a biphasic waveform of 3 ms/3 ms. The model 3020 defibrillator had a 160-µF capacitor with a maximal shock of 6J with a biphasic waveform of 6 ms/6 ms.
The coronary sinus (CS) and right atrium (RA) leads were innovative single-electrode designs that both sensed and transmit shock. The CS lead had a tip with a helical twist that fixes it in place and prevents blood flow from pushing it back out. During insertion, a center wire kept the lead straight. Once positioned, the wire was removed and the lead curled into its final shape.
From the engineering perspective, the Metrix device was truly innovative. A 1997 article by Mark Gottschalk in Design News (Atrial Defibrillator Tackles Matter of the Heart) described some of its novel features:
“Power for the Metrix unit is supplied by a lithium-silver-vanadium-oxide battery. To produce the cardioversion shock, a maximum 300-volt, 6-joule burst, the battery charges a 160-microfarad, aluminum-electrolytic capacitor of the type used in camera flashes. The capacitor passes the current to several FETs that deliver to the heart, on cue from the system, a bi-phasic waveform–6 msec on one polarity and 6 msec on the other.
…
A custom circuit board holds two ICs developed in-house, the microprocessor, and static RAM. One of the units developed in-house, a digital IC, manages operations such as the high-voltage control logic and telemetry interface. The second, an analog IC, handles such items as the three ECG channels and the 8-bit D/A converter. Supplied by Philips Components-Signetics (Sunnyvale, CA), the microprocessor is a variation of the 8051 and runs at 2 MHz on just 1.8 volts. One megabyte of SRAM (8 × 128k) stores the unit’s program as well as ECG, performance, and therapy data.
Engineers took some unique approaches to reduce Metrix’s power drain and extend battery life. “The challenge was to put all this intelligence in the device and yet have it last four years and draw typically less than 20 microamps,” says Phillip Foshee, design engineering director.
Metrix can be operated in automatic, manual, and physician-activated modes. But even in automatic mode, it is almost completely off more than 95% of the time. At programmed intervals–say 20 minutes–a timer turns on the CPU so the unit can collect ECG data and analyze it. If the heart is in normal rhythm, Metrix goes back to sleep. To allow the unit to be awakened on demand, the telemetry system continuously looks for a signal from the InControl programmer unit by powering up for 60 µsec every 125 msec.
The telemetry system communicates with the external InControl Programmer via a 2-MHz carrier wave that can simultaneously transmit digital data for the three ECGs as well as status and command information. “We can telemeter the contents of the 1-meg static RAM in four seconds,” says Foshee. This performance represents as much as a factor of ten speed improvement over other implantable devices that often take minutes to transmit far less data. Engineers achieved the high transmission speed by removing the telemetry coil from inside the metal case and placing it in the plastic header.”
The device could be programmed in an automatic mode with automatic, periodic activation of the detection algorithm and shock delivery after a preset delay from onset of AF, or it could be used in a patient- or physician-activated mode in which the detection algorithm and shock therapy were initiated by placing a magnet over the Metrix device.
In 1998 Guidant (now Boston Scientific) acquired InControl and promptly closed its operations. The stand-alone implantable atrial defibrillator did not achieve acceptance, mostly because of lack of patient tolerance to the device-based atrial therapy for AF. The discomfort produced by the atrial shock without the use of sedation was the device’s major limitation.
Click here for scanned copy (1MB .pdf) of M.A. Gottschalk, “Atrial Defibrillator Tackles Matter of the Heart”, Design News, 65-66, 6/9/1997. Click here for full text from Design News.
Click here for InControl’s patents