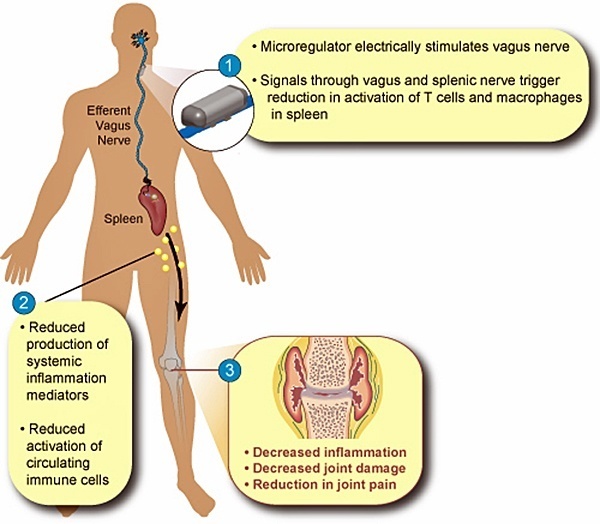
SetPoint Medical, headquartered in Valencia, California, is developing neuromodulation therapies for patients with inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, diabetes, heart disease, and multiple sclerosis. SetPoint’s proprietary neuromodulation platform consists of an implantable “microregulator”, wireless charger and iPad prescription pad application.

 Toronto-based
Toronto-based 

 I received today a
I received today a 
 Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.



 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.