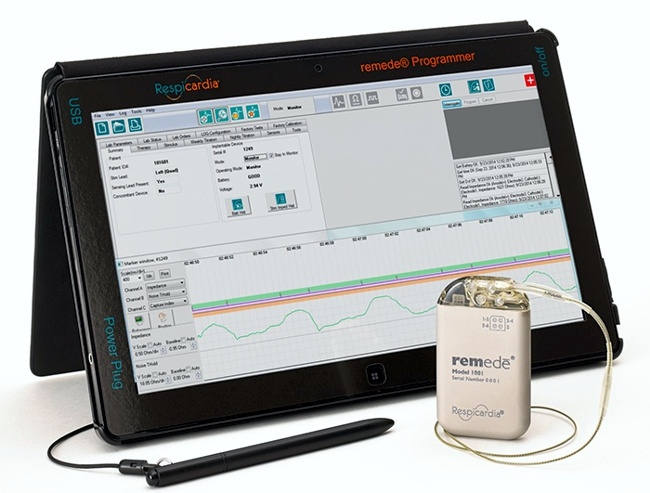
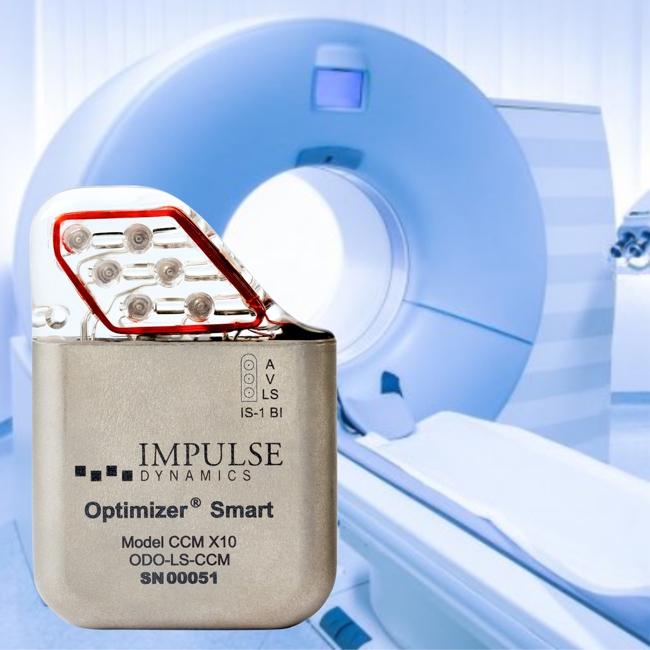
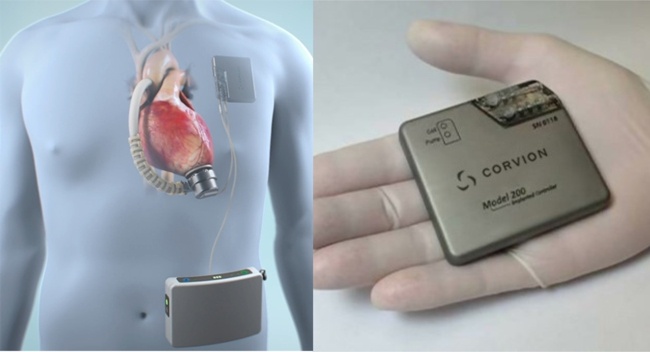
FDA published yesterday a final guidance document which provides recommendations on testing to assess the safety and compatibility of medical devices in the Magnetic Resonance (MR) Environment and the recommended format for Magnetic Resonance Imaging (MRI) Safety Information in medical device labeling.
This new guidance supersedes FDA’s 2014 guidance “Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment.” The new guidance applies to all medical devices that might be used in the MR environment. This includes all implanted medical devices, medical devices that are fastened to or carried by a patient (e.g., external insulin pump, pulse oximeter), medical devices that would reasonably be anticipated to enter the MR environment during clinical care, and all medical devices that are intended to enter the MR environment.
For active implantable medical device (AIMDs), the guidance makes reference to ISO/TS 10974 “Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device.”

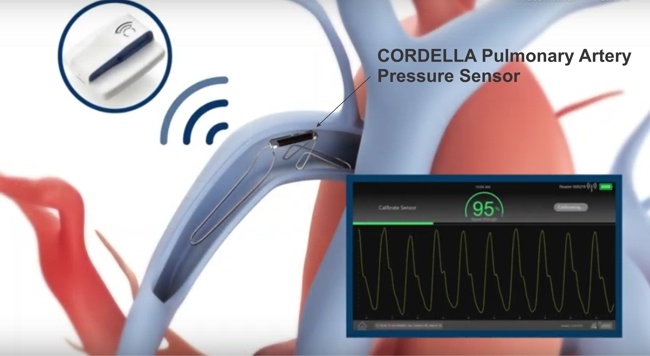
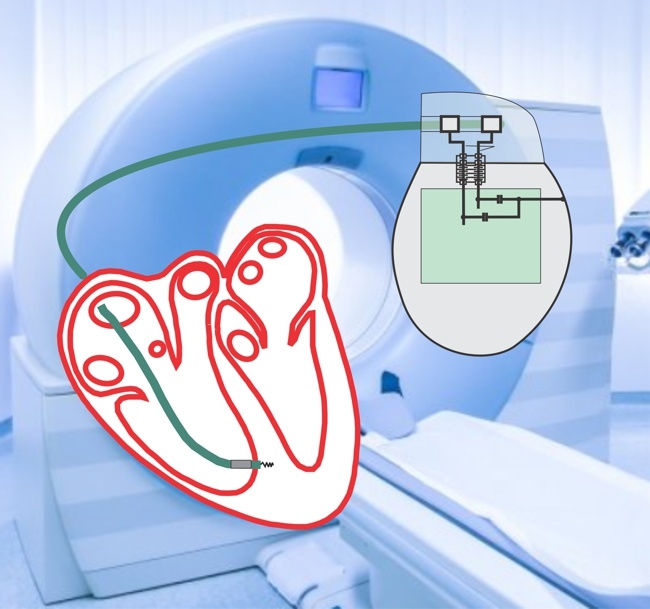



 Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.
Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.