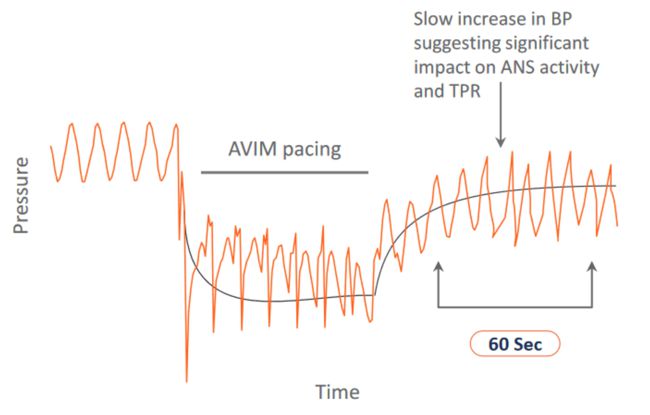
Image Credit: Orchestra BioMed
Orchestra Biomedical announced that it has received FDA’s approval (Sep 19, 2023) to initiate its BACKBEAT (BradycArdia paCemaKer with atrioventricular interval modulation for Blood prEssure treAtmenT) IDE study for evaluating the efficacy and safety of atrioventricular interval modulation (“AVIM”) therapy (also known as BackBeat CNT™) for treating hypertensive patients who are indicated for a dual-chamber cardiac pacemaker.
Orchestra and Medtronic formed a strategic collaboration for the development and commercialization of AVIM therapy for hypertensive pacemaker patients in July 2022. Under the collaboration, Medtronic is providing Orchestra BioMed with development, clinical, and regulatory support for the BACKBEAT global pivotal study, which Orchestra BioMed is sponsoring. If approved, Medtronic will have exclusive global rights to commercialize AVIM-enabled pacing systems for this target population.