Image Credit: Biotronik
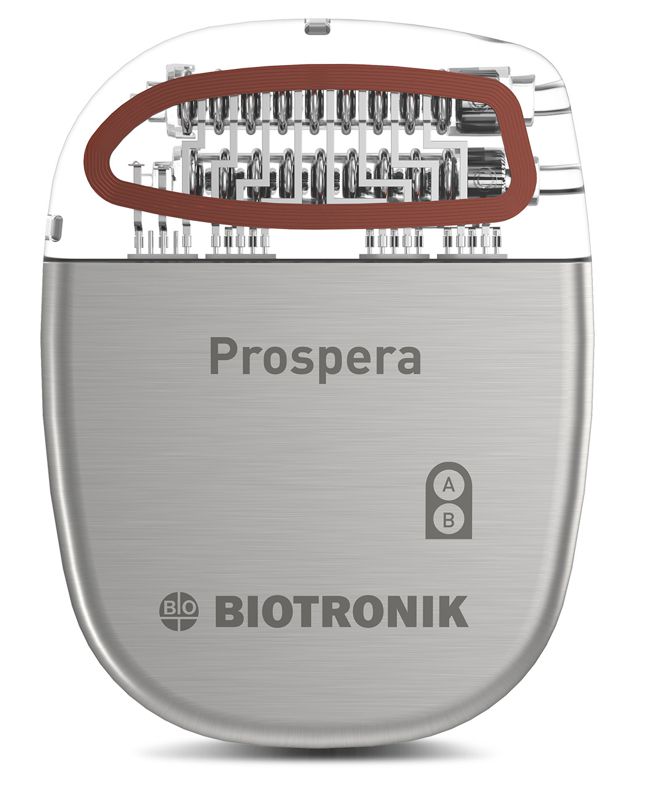
Biotronik received FDA approval for its Prospera™ Spinal Cord Stimulation System on March 31, 2023. The system is intended to treat chronic, intractable pain in the trunk or limbs. The Prospera SCS System includes a rechargeable IPG, SCS leads, a clinician programmer, a patient programmer, and an external trial pulse generator.
This weekend at NANS 2025 Biotronik presented 24-month intermediate data from its BENEFIT-03 prospective, multicenter clinical study in Australia. Continue reading







 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD. Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.