
The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.

The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.

My friend Daniel Villamil from CCC Medical in Uruguay sent me these pictures of a very unique device in his colection. It is a late-1960s/early 1970s pacemaker made in Sao Paulo, Brasil.
UPDATE Oct 3, 2012:
CCC’s CEO Julio Arzuaga recalled that this pacemaker was manufactured in the early 1960s by the Instituto de Cardiologia Dante Pazzanese in Sao Paulo, Brasil. The physicians leading the pacemaker team were Dr. Decio Kormann and Dr. Adib Jatene.
Dr. Orestes Fiandra used to implant these Brasilian pacemakers in Uruguay. However, they were not very reliable. For this reason, and with help from Drs. Kormann and Jatene, Dr. Fiandra started CCC del Uruguay as a more industrial environment for the production of pacemakers.

This is a picture of the first pacemaker to be implanted in a human patient. It was developed by Dr. Rune Elmqvist (1906–1996), a physician by training, but working for the Swedish company Elema-Schonander as an engineer. Dr. Elmqvist developed the device in cooperation of Åke Senning, senior physician and cardiac surgeon at the Karolinska University Hospital in Solna, Sweden. Continue reading→
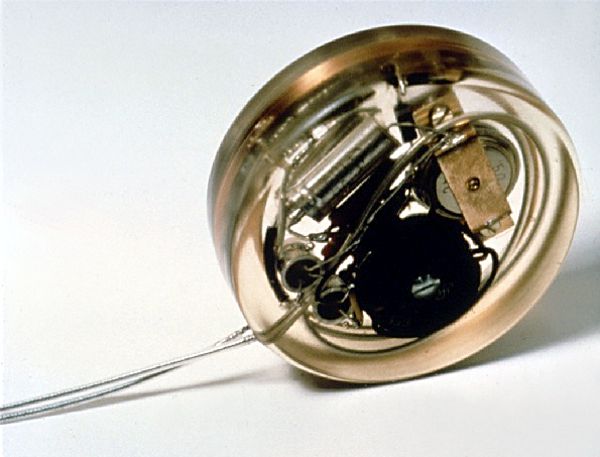
This nuclear pacemaker was manufactured ca. 1972 by Dr. Orestes Fiandra’s CCC del Uruguay. It was powered by a McDonell-Douglas Betacel 400 which had promethium-147 sandwiched between semiconductor wafers. As the radioactive promethium isotope decays, it emits β-particles (electrons). The impact of the β-particles on a p-n junction causes a forward bias in the semiconductor similar to what happens in a photovoltaic cell (a solar cell).
The Betacel 400 had an open-circuit voltage of 4.7V and a short circuit current of 115μA. The maximum power output was 370μW. CCC’s pacemaker was expected to last for 10 years when powered by this nuclear battery. Continue reading→

Shree Pacetronix Ltd., was founded in Pithampur, Dist. Dhar, India in 1988. In 1993 the company was converted to a Public Limited Company. The company is listed on the Bombay Stock Exchange and regional exchanges.
Pacetronix’s website shows EC type certificates for its Pinnacle SSIR (model 297), Pinnacle SSI (model 8820), Charak DDD (model ND 747), and Akash VDD (model ND 244) pacemakers. Continue reading→
This is one of my most prized possessions. It is one of the very first pacemakers produced by CCC del Uruguay in 1969. It was given to me by my friend, the late Dr. Orestes Fiandra, founder of CCC del Uruguay.
On February 2, 1960, Dr. Orestes Fiandra and Dr. Roberto Rubio accomplished the first succesful long-term human implant of a pacemaker. The pacemaker was manufactured by Dr. Rune Elmqvist of Elema-Schönander in Sweden, and was implanted in Uruguay in a 34-year-old patient with AV block. This unit worked successfully for nine and a half months, until the patient died of sepsis from an unrelated infection.
In 1969, Dr. Fiandra started the “Centro de Construccion de Cardioestimuladores del Uruguay” (CCC for short) with the purpose of producing pacemakers for use in Latin America at prices well under those of American devices. The device in the photograph above is one of these devices – a simple VOO pacemaker powered by 5 mercury cells encapsulated in epoxy resin.
 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.This was achieved in Uruguay on February 2, 1960 by Dr. Orestes Fiandra and Dr. Roberto Rubio. The pacemaker was manufactured by Dr. Rune Elmqvist of Elema-Schönander in Sweden, and was implanted in Uruguay in a 34-year-old patient with AV block. This unit worked successfully for nine and a half months, until the patient died of sepsis from an unrelated infection. Continue reading→