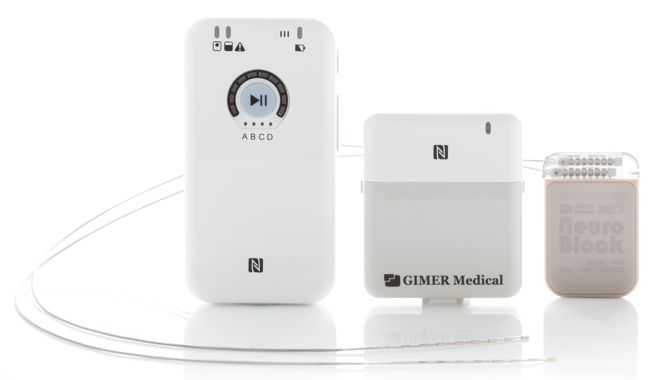
Image Credit: Gimer Medical
Gimer Medical is a Taiwanese medical device manufacturer founded in 2013. It set up the first implantable electronics factory in Taiwan in 2018.
Gimer’s NeuroBlock spinal cord stimulation (SCS) system is based on technology developed at the Taiwan National University in 2008 combining pulsed radio frequency (PRF) with SCS into the so-called “+RF” technology – an ultra-high frequency of 500 kHz, which Gimer claims has a high penetration rate, no heat sensation during the treatment, no protein denature, and no action potential changes. It is expected to produce paresthesia-free and prolonged treatment effect for chronic pain patients.
On August 13, 2022, the US FDA granted conditional Investigational Device Exemption (IDE) approval to Gimer to trial the NeuroBlock SCS system.
