Image Credit: Mainstay Medical
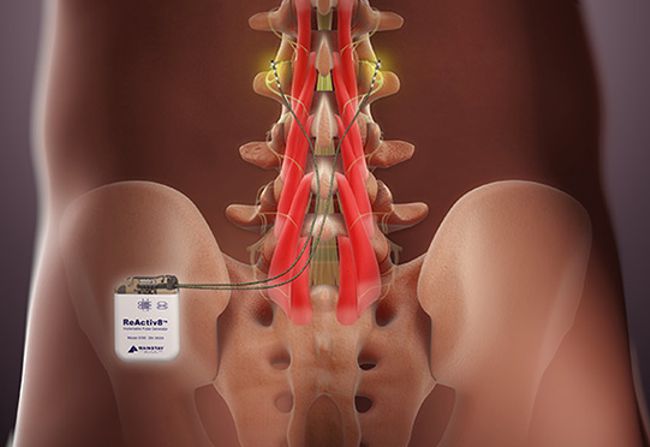
Dublin, Ireland-headquartered Mainstay Medical announced that FDA has approved its ReActiv8 neurostimulator is intended to treat chronic low back pain caused by arthrogenic muscle inhibition. This is a neuroprotective condition in which the brain reflexively attempts to limit motor stimulation and movement in muscles surrounding a joint in response to damage or injury to the joint. In patients with this type of back pain, an initial injury triggers this reflexive inhibition, and the brain tries to limit painful movement by altering nerve transmissions to the deep stabilizing muscles of the spine, causing a loss of motor control, an unstable spine, and greater susceptibility to reinjury.
According to Mainstay, arthrogenic muscle inhibition causes the vicious cycle of recurring pain, instability, and reinjury commonly seen in patients with chronic nonspecific low back pain. The ReActiv8 System attempts to interrupt this cycle by using neurostimulation to reactivate the motor control system driving the deep stabilizing muscles of the spine. Stimulation is delivered via two leads which are placed bilaterally near the medial branch of the dorsal ramus nerve at the L3 vertebra. An external wireless activator is used to start and stop stimulation sessions typically for 30 minutes twice a day.
According to the announcement:
“The FDA approval grants Mainstay the right to market ReActiv8 in the United States as an aid in the management of intractable chronic low back pain associated with multifidus muscle dysfunction, as evidenced by imaging or physiological testing in adults who have failed therapy, including pain medications and physical therapy, and are not candidates for spine surgery.”

![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)