Image credit: MicroTransponder
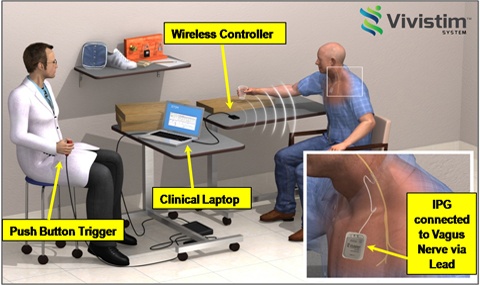
FDA approved today the MicroTransponder Vivistim Paired VNS System, a first-of-its-kind, drug-free rehabilitation system intended to treat moderate to severe upper extremity motor deficits associated with chronic ischemic stroke using vagus nerve stimulation (VNS).