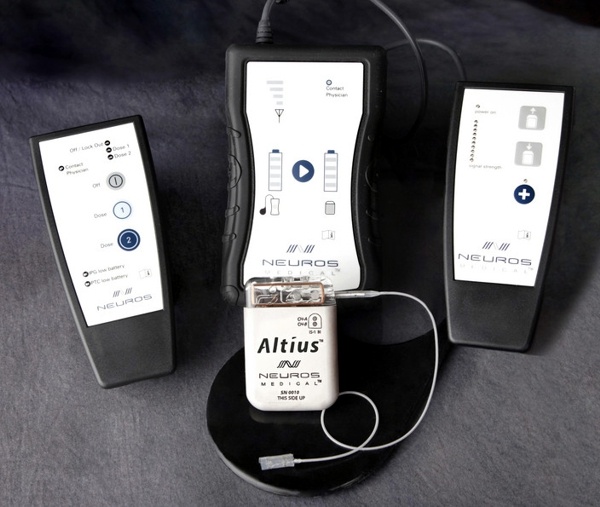
Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.
Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.