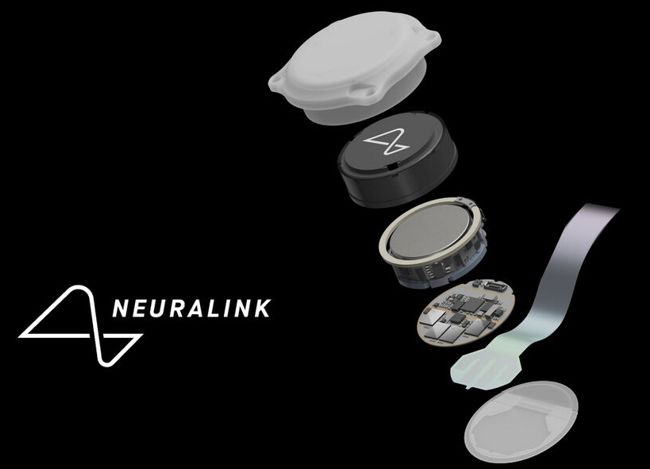
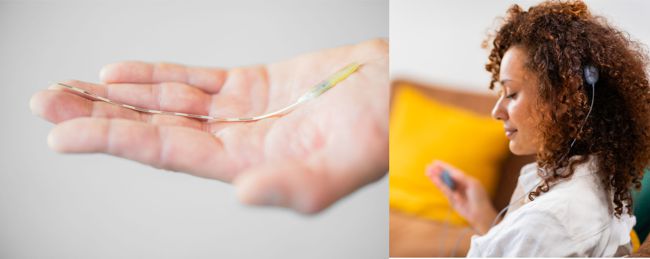
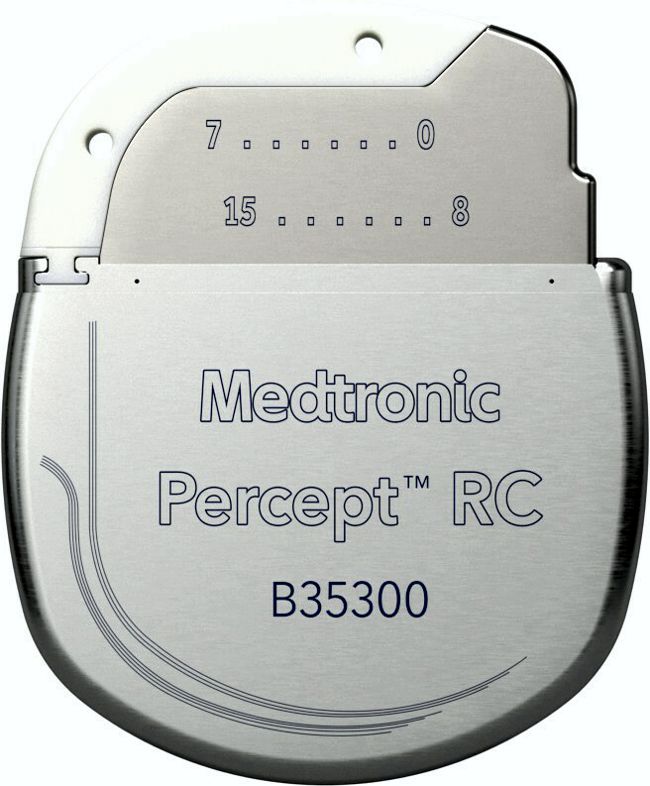
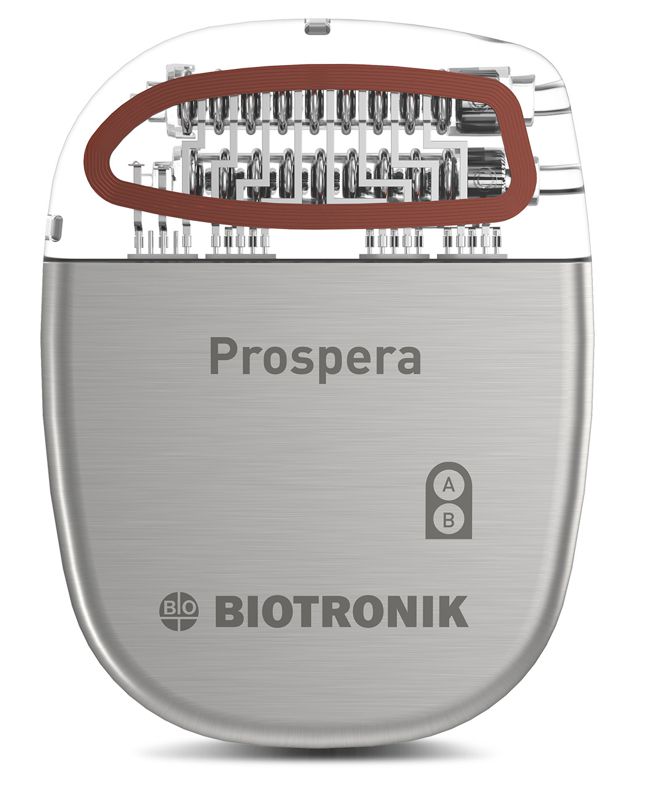
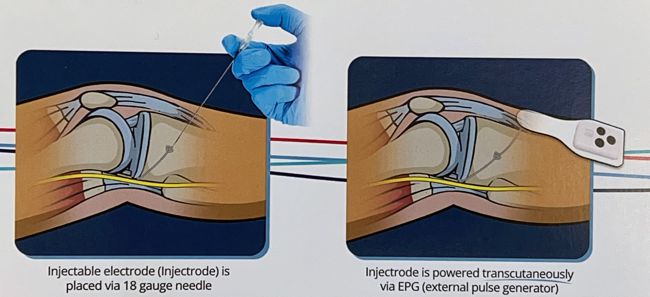
Image credit: Impulse Dynamics
Impulse Dynamics, the company where I’m an Exec VP and CTO, announced today the completion of enrollment in the INTEGRA-D trial of the CCM-D system intended for the treatment of heart failure.
From the press release:
“The company successfully completed enrollment in the INTEGRA-D™ Pivotal Trial. This marks a significant milestone in the journey to introduce the CCM-D® HF System, the world’s first device combining Impulse Dynamics®’ proprietary CCM® therapy for the treatment of HF symptoms with lifesaving, implantable cardioverter defibrillator (ICD) therapy. The trial’s initial results, including 100 percent defibrillation success in the efficacy cohort, were presented as a late-breaking clinical trial at Heart Rhythm 2025 and support a forthcoming PMA submission to the FDA. This milestone advances thepotential approval of a first-of-its-kind dual therapy that is designed to address both sudden cardiac death and chronic HF symptoms.”