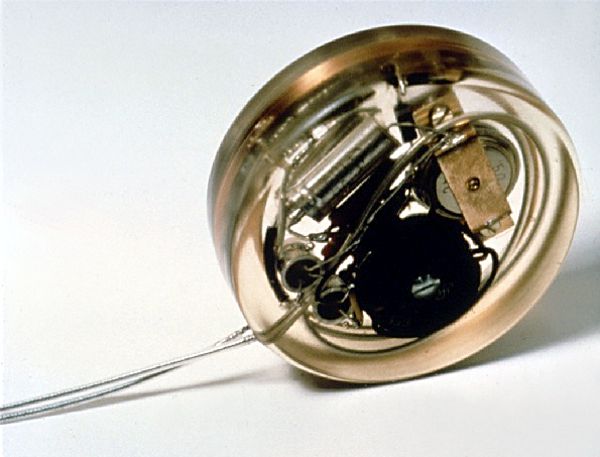
This is a picture of the first pacemaker to be implanted in a human patient. It was developed by Dr. Rune Elmqvist (1906–1996), a physician by training, but working for the Swedish company Elema-Schonander as an engineer. Dr. Elmqvist developed the device in cooperation of Åke Senning, senior physician and cardiac surgeon at the Karolinska University Hospital in Solna, Sweden. Continue reading














 One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).
One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).