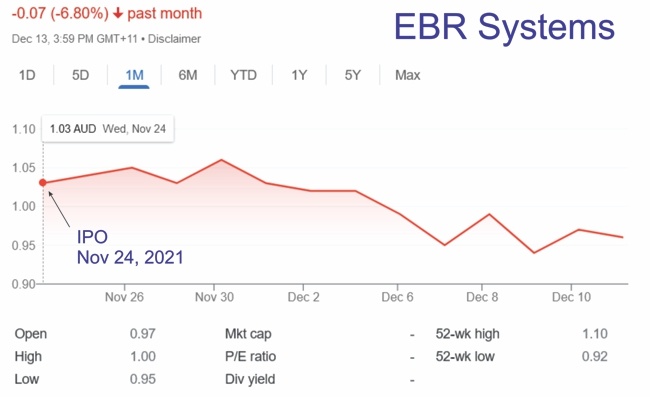
On November 24, 2021, EBR Systems went public in the Australian stock exchange. According to the press release, the IPO raised AU$110M (around $78.5M). EBR pland to use these funds to complete its pivotal study, targeting FDA submission for approval in 2023 followed by rapid U.S. commercial launch.