
In October 2016, St. Jude Medical (now Abbott) issued an advisory on a family of ICD and CRT-D devices that may develop Lithium deposits within the battery leading to a short circuit and result in premature and potentially rapid battery depletion. However, according to the DOJ, St. Jude was already aware of the issue by 2013. In 2014 St Jude asked the FDA to approve an update to the devices to mitigate the battery issue. In its request, St. Jude claimed that the issue had not caused any serious injuries or deaths, but the DOJ claimed that the company had been informed of two injuries and one death caused by the issue.
The lawsuit covers the period between November 2014 and October 2016, during which the DOJ claims St. Jude was fully aware of the device’s issues but continued selling thousands of devices. By August 2016, St. Jude reported to FDA two more deaths linked to this issue, and over 700 cases of premature battery depletion.
The federal case was brought under the False Claims Act based on the allegation that by continuing to sell the potentially harmful products to healthcare facilities and implanted in patients enrolled in Medicare and Medicaid, St. Jude played a role in securing government payments for devices that they knew were defective. It was announced on July 8, 2021 that St. Jude Medical agreed to pay $27 million to settle this case.



![IMAGE_Nanostim_Product_Shot[1]](https://www.implantable-device.com/wp-content/uploads/2013/10/IMAGE_Nanostim_Product_Shot1.jpg)

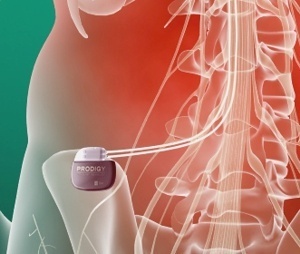 St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.
St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.

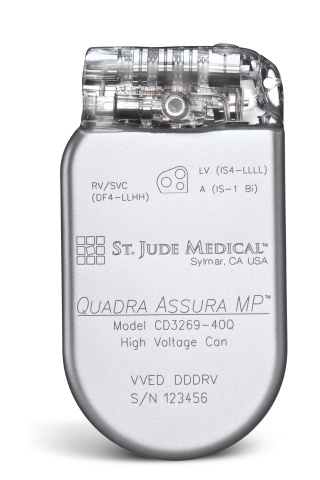 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
 St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.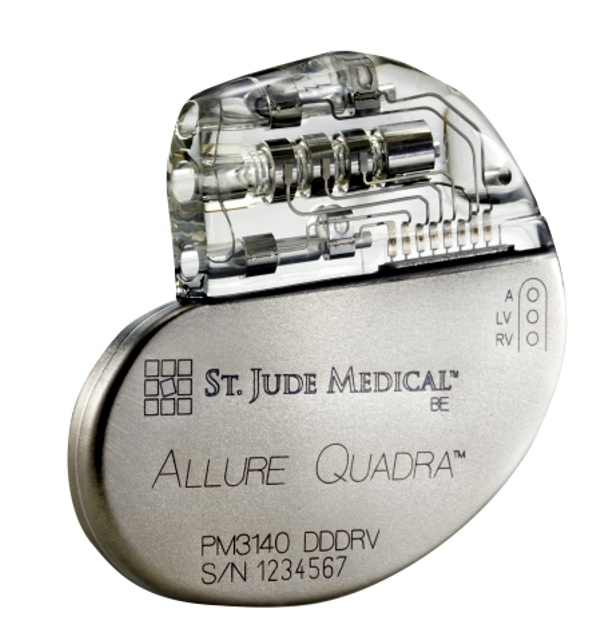 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release: