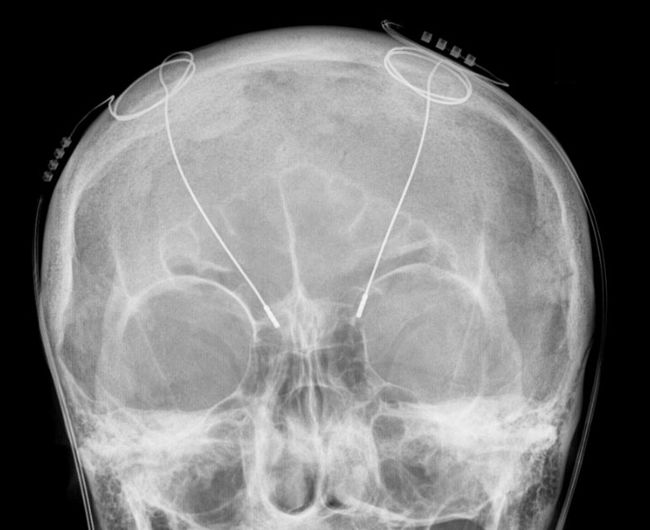
Image credit: Modified from https://commons.wikimedia.org/wiki/File:Tiefe_Hirnstimulation_-_Sonden_RoeSchaedel_ap.jpg
I attended the “Engineering Principles of Deep Brain and Spinal Cord Stimulation” workshop at NANS 2025, and was very impressed by Dr. Cameron McIntyre’s presentation where he analyzed the state of the DBS market.
Dr. McIntyre showed that the cost of developing DBS technology over the past two decades was justified by the expected market growth. However, the DBS market is not growing anywhere near the rate at which it was expected. In 2024 it had barely returned to 2019 numbers after it sharply decreased during COVID, and is now projected to stay flat for years to come.
The existential threats to DBS are:
- Anemic growth,
- High-Intensity Focused Ultrasound (HIFU),
- Infusions, and
- “Magical” biologics
The main issue is that although DBS is the best therapy for Parkinson’s, the vast majority of patients refuse it because they feel that it is too invasive. Instead, they are lured to High-Intensity Focused Ultrasound (HIFU) because, although in Dr. McIntyre’s view it is a”silly concept,” it promises to be a quick fix.
In Dr. McIntyre’s view, if DBS doesn’t find new indications, investment will shrink and its market will drop to half within 5 years. These new indications could include epilepsy, depression, and stroke.
Another major factor that is causing DBS’ decline is its currently overcomplicated clinical workflow. Dr. McIntyre believes that in order to survive, a major paradigm shift is needed to standardize DBS surgery and automatize DBS management.