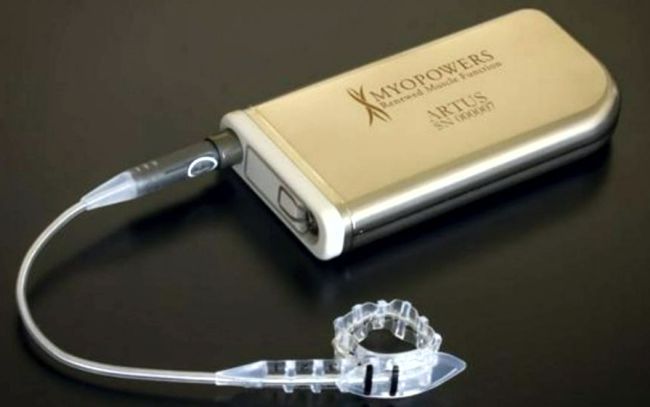
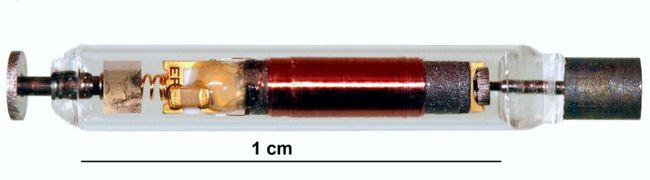
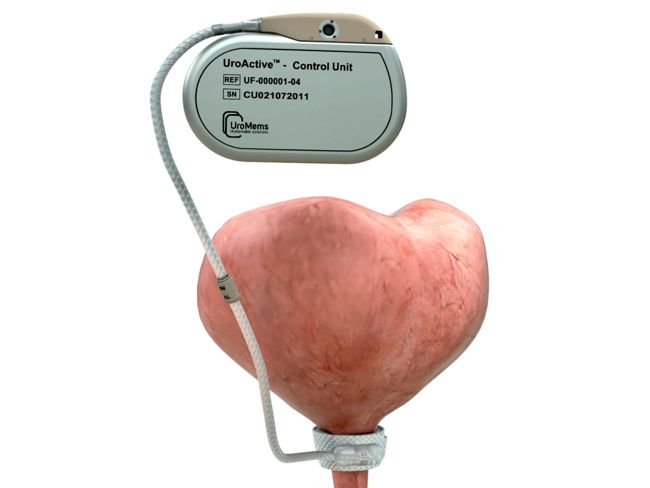
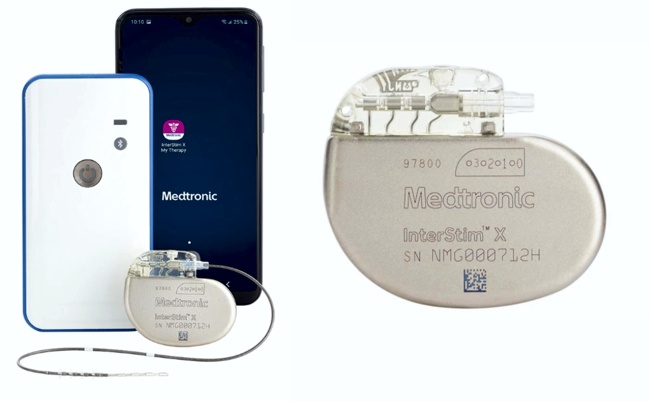
Image Credit: Axonix
Medtronic and Boston Scientific have reached a confidential settlement over the IP dispute between Medtronic and Axonics (now part of Boston Scientific). The settlement, which includes a payment from Boston Scientific to Medtronic resolves all outstanding litigation between Medtronic and Axonics.