
Image Credit: Zamora et al https://doi.org/10.1101/2020.09.10.292284
UK news outlets reported that a Picostim deep-breain stimulator made by UK company Bioinduction (which was recently acquired by Amber Therapeutics) was recently implanted in a boy with Lennox-Gastaut syndrome, which is a a severe, treatment-resistant form of epilepsy that he developed at the age of three.
The implant was performed at the UCL Great Ormond Street Institute of Child Health & Great Ormond Street Hospital under the “CADET” project (Children’s Adaptive Deep brain stimulation for Epilepsy Trial). According to the project’s website, the Picostim system is especially suitable for DBS therapy in this application:
-
“The advanced capabilities of Picostim DyNeuMo-1 allow for personalised and adaptive stimulation regimes according to seizure patterns and circadian (wake/sleep) rhythms at an individual patient level, which are key components of LGS symptoms. These capabilities are here proposed to maximize the potential of DBS to limit seizure spread, and reduce seizure frequency and severity.
-
These capabilities are made possible by the ability to non-invasively and repeatedly recharge the device. Patients or parents are able to use a handheld or headset-mounted charger that safely recharges the battery through the skin.
-
The device allows for the recording of electrical activity in the implanted brain, potentially allowing stimulation parameters that are tailored to LGS and to the individual child.
-
Lastly, the whole device is mounted to the skull, removing the need for (painful) tunneling of extension leads to a battery implanted in the chest wall and potentially reducing the risk of infection.”
The CADET pilot (Children’s adaptive deep brain stimulation for epilepsy trial) will now recruit three additional patients with Lennox-Gastaut syndrome, with a view to 22 patients being recruited to take part in a full trial.
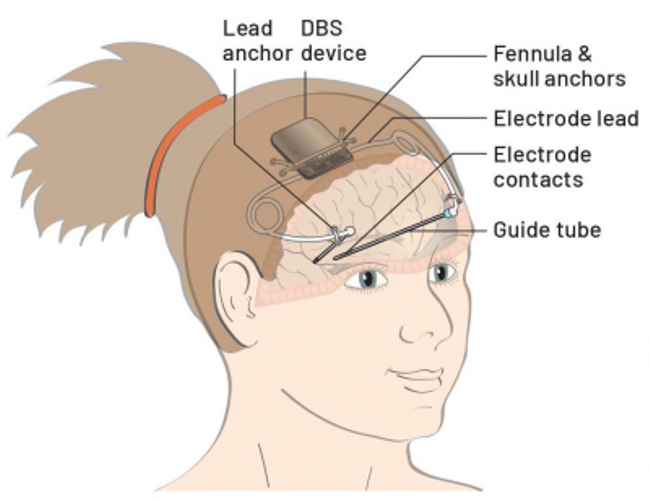
Image Credit: CADET Project, UCL Great Ormond Street Institute of Child Health
