
In the late 1960s Medtronic – today the largest manufacturer of implantable medical devices in the world – teamed up with Alcatel, a French company, to design a nuclear-powered pacemaker. The first human implant of the device took place in Paris in 1970.
The nuclear battery in the Medtronic device used a tiny 2.5 Ci slug of metallic Plutonium 238 (Pu-238). The radiation produced by the Pu-238 bombarded the walls of its container, producing heat that a thermopile then converted to an electrical current. A thermopile is a stack of thermocouples, which are devices that convert thermal energy directly into electrical energy using Seebeck effect. A thermocouple is made of two kinds of metal (or semiconductors) connected to each other in a closed loop. If the two junctions are at different temperatures, an electric current will flow in the loop. Continue reading

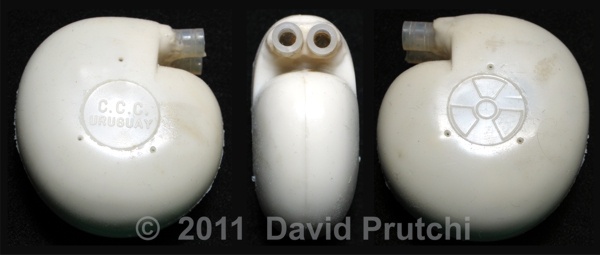



 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.

 Intrapace was founded in Mountain View, CA by
Intrapace was founded in Mountain View, CA by  Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.
Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.


 Second Sight Medical Products, Inc., located in Los Angeles, CA, was founded in 1998 by Alfred Mann to develop a retinal prosthesis to provide sight to patients blinded from outer retinal degenerations, such as Retinitis Pigmentosa.
Second Sight Medical Products, Inc., located in Los Angeles, CA, was founded in 1998 by Alfred Mann to develop a retinal prosthesis to provide sight to patients blinded from outer retinal degenerations, such as Retinitis Pigmentosa.