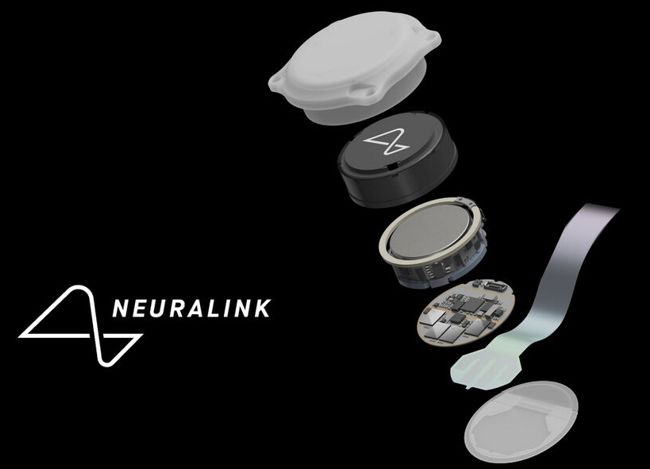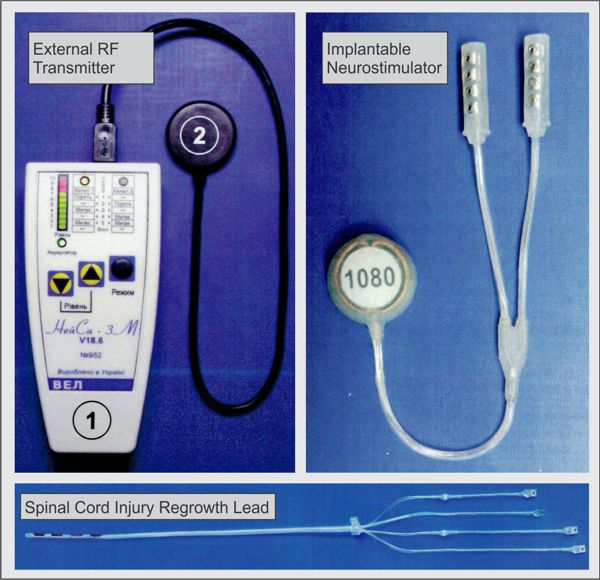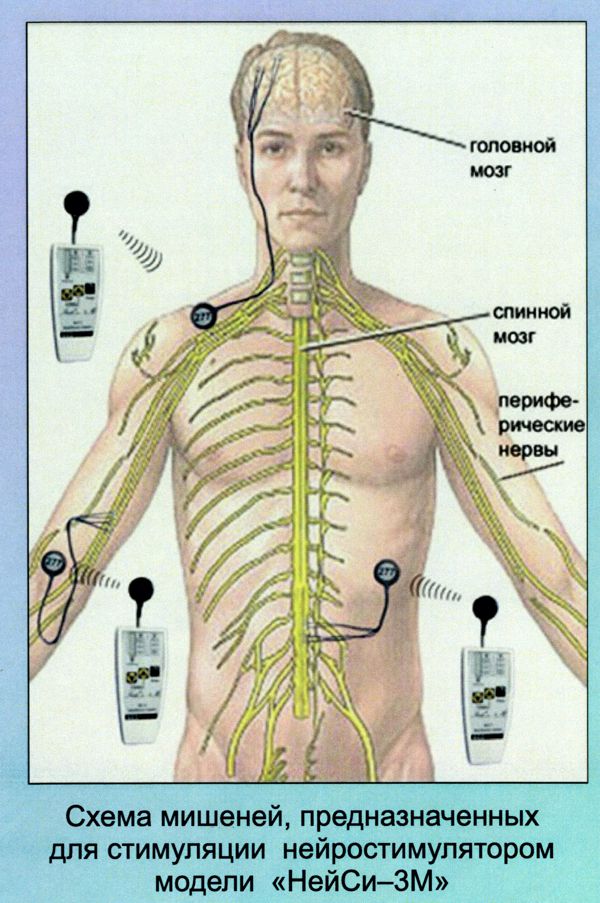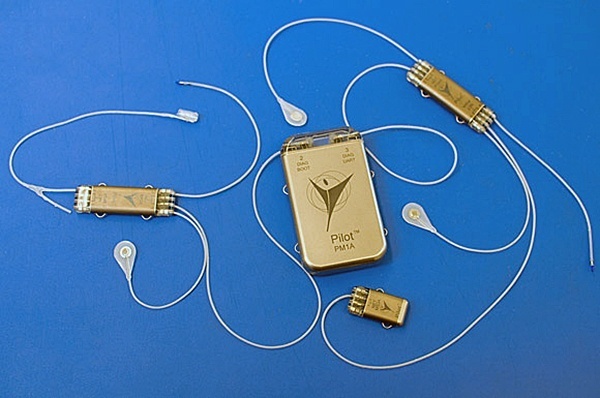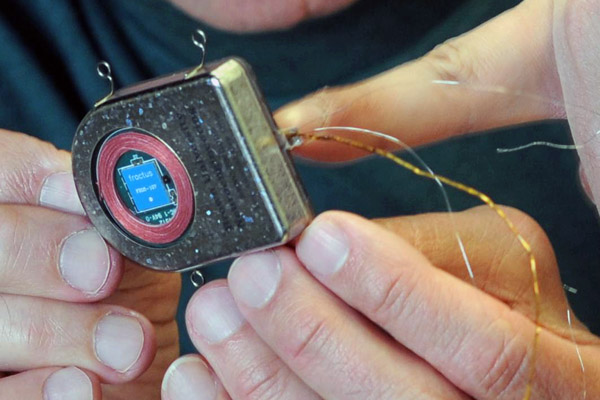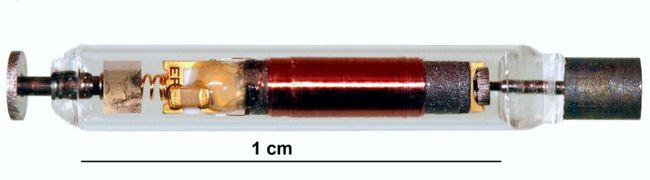
First-generation RF-powered BION (RFB1) microstimulator. Image Credit: Alfred Mann Institute for Biomedical Engineering
In 1991, Dr. Gerald Loeb, at the time a Professor of Physiology and Biomedical Engineering at Queen’s University (Kingston, Canada), first proposed a miniature, injectable, RF-powered device for the stimulation of tissue or motor neurons. The BION® device was developed based on this concept as a joint project between Queens University (Kingston, ON, Canada), IIT (Chicago, IL), and the Alfred E. Mann Foundation (Valencia, CA) with funding from the NIH Neural Prosthesis Program. The RF BION 1 (RFB1) was then manufactured by the Alfred Mann Institute for Biomedical Engineering at USC.