
The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.

The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.

Image Credit: CARSS
The University of Southern California, Medipace Inc., and Med-Ally LLC are the CARSS collaborative team part of NIH’s Human Open Research Neural Engineering Technologies (HORNET) initiative for open-architecture, open-source implantable neuromodulation system. This team is developing the OpenNerve implantable neuromodulation platform, which consists of an IPG with an associated charging device. The OpenNerve IPG will perform current-based neuromodulation, measure impedance, monitor electronic biosignals, and interface with chemical and physical sensors.
All files relevant to the design and manufacture of the CARSS OpenNerve Platform are available on a Github repository. Details are posted on the project’s Wiki.
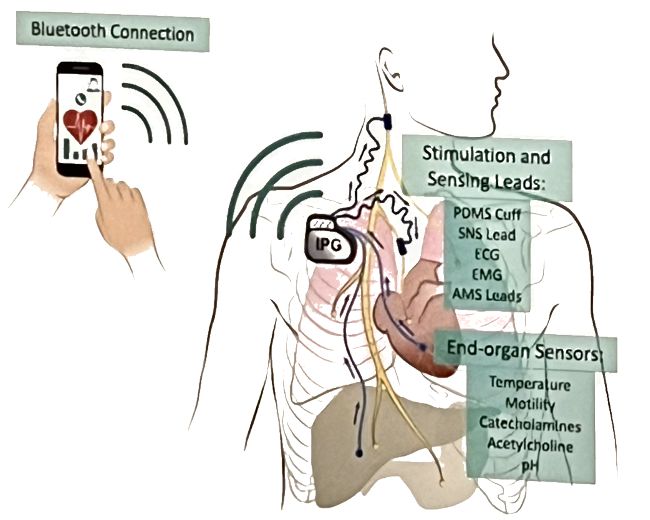
Image Credit: CARRS

Image Credit: Nuvectra
Back in 2013, implantable-grade component manufacturer Greatbatch Medical (now Integer Holdings) briefly entered the finished devices market with its own Algostim Spinal Cord Stimulator.
However, when Greatbatch acquired CCC Medical in 2014, having the Algostim SCS was seen as a conflict of interest by some of CCC’s SCS customers (e.g. Nevro), so in 2015/2016 Greatbatch divested its SCS business into a new company called Nuvectra.
Nuvectra never caused much of an impact in the SCS market, and trailed well behind Abbott, Boston Scientific, Medtronic, and Nevro. After limping along for a number of years, on November 12, 2019, Nuvectra filed for Chapter 11 bankruptcy.
Cirtec Medical, a competitor of Integer’s CCC in the development of implantable devices, acquired Nuvectra’s IP and assets. Cirtec intends to leverage this technology to support customers’ development of neuromodulation products, providing an FDA approved platform with a complete design history file for a variety of therapeutic applications.
The Nuvectra assets acquired by Cirtec include all owned intellectual property associated with the SCS, SNS, and DBS platforms developed by Nuvectra.

ASIC designer NanoWattICs (Uruguay) and Rosellini Scientific (Dallas, TX) have announced they have entered into a collaborative relationship for the development of a suite of neurostimulation devices comprising implantable, wireless and non-invasive technologies. From the press release:
Rosellini Scientific, LLC (“Rosellini Scientific”; Dallas, TX, USA) and NanoWattICs SRL (“NanoWattICs”; Montevideo, UY) are pleased to formally announce their collaborative working relationship. For the past twelve months, NanoWattICs has provided support for the engineering efforts required by portfolio companies belonging to Rosellini Scientific.
Rosellini Scientific and NanoWattICs share common developmental interests and possess complementary strengths and expertise, which has produced a fruitful collaboration to date. The continuation of this long-term alliance will allow NanoWattICs to continue growing its team while participating in the development of innovative medical technologies guided by Rosellini Scientific.
“NanoWattICs are world-class engineers striving to develop the next generation of innovative medical technologies,” said Austin Duke, Director of Emerging Therapies at Rosellini Scientific. “We believe that combining their engineering expertise with the Rosellini Scientific vision will greatly enhance the process of bringing high-quality, life-changing medical technologies to the global market.”
“We are extremely pleased and excited about this collaboration opportunity with Rosellini Scientific. The combined knowledge of the two teams can hardly be matched by other players of our size in the industry,” said Pablo Aguirre, Director and Co-Founder of NanoWattICs. “We look forward to expanding our team and together bringing to market the next generation of medical devices.”
Currently, Rosellini Scientific and NanoWattICs are developing a suite of neurostimulation devices comprising implantable, wireless and non-invasive technologies. This work is being supported in part by a recently awarded grant funded by Agencia Nacional de Investigación e Innovación (ANII) in Uruguay. The devices are being developed for a variety of clinical indications, including cardiac arrhythmias, migraines and neuropathic pain.
Previously, NanoWattICs provided engineering support for Rosellini Scientific to assist in the development of a non-invasive medical imaging system (Spectral MD Inc.) and wireless telemetry capabilities for a novel glaucoma implant (collaborative development with ISTAR Medical SA).
UPDATE Jan 19, 2017: Rosellini Scientific merged with Nexeon MedSystems

Image Credit: Micro Systems Technologies
Micro Systems Technologies (MST) is the vertically-integrated supplier of microelectronics and implantable-grade components to Biotronik. It now offers its development and manufacturing services to others.
Through its companies, MST offers high-reliability microelectronic modules for implantable medical devices such as pacemakers, defibrillators, neurostimulators, and cochlear implants.
MST can provide integrated solutions encompassing everything from conceptual design through high-volume surface-mount (SMT) assembly of modules. Services include design development of customer-defined electronic modules, custom ASIC design and development, and high-density-interconnect (HDI) substrate and assembly design.
As a OEM, MST offers state-of-the-art automated SMT line, an ISO 13485–compliant process and component verification and validation methodology, a manufacturing execution system that automates data collection, analysis, and process control, and comprehensive supply-chain management and monitoring with 100% traceability. Continue reading→

 Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.
Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.
Nanowattics’ core team is extremelly experienced in the design of ASICs for implantable medical devices. Their designs include the main ASIC for a DDDR pacemaker, a sub-microvolt differential amplifier for electroneurographic signal acquisition, and the chipset for a multi-channel current-mode implantable stimulator.
Besides IC design services, Nanowattics also offers IC development project management through the entire product design cycle, including feasibility studies, translation of requirements, foundry submission, fabrication, packaging, prototype testing, documentation, certifications, and production. Continue reading→
 We conduct reliability analyses for our implantable devices on a continued basis. I’ve spent the last few days readying the data for this period’s analysis, and thought that a short primer on how this is actually done would be of interest to fellow engineers who may need it at some point.
We conduct reliability analyses for our implantable devices on a continued basis. I’ve spent the last few days readying the data for this period’s analysis, and thought that a short primer on how this is actually done would be of interest to fellow engineers who may need it at some point.
You surely have heard of MTBF = Mean Time Between Failures. This is a key reliability metric. However, since our implantable devices are single-use, the first failure is the final failure, so MTTF = Mean Time To Failure. As such, for a device with no possibility of repair (e.g. implantables, satellites), MTBF=MTTF. Continue reading→

EBR Systems, Inc., founded in 2003 and headquartered in Sunnyvale, CA, is developing the WiCS® Wireless Cardiac Stimulation technology to eliminate cardiac pacing leads, historically a major source of complications and reliability issues. The startup was spun out of research by founder Debra Echt, a former professor of medicine and a cardiologist at Vanderbilt University. Continue reading→

Shree Pacetronix Ltd., was founded in Pithampur, Dist. Dhar, India in 1988. In 1993 the company was converted to a Public Limited Company. The company is listed on the Bombay Stock Exchange and regional exchanges.
Pacetronix’s website shows EC type certificates for its Pinnacle SSIR (model 297), Pinnacle SSI (model 8820), Charak DDD (model ND 747), and Akash VDD (model ND 244) pacemakers. Continue reading→
 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.This was achieved in Uruguay on February 2, 1960 by Dr. Orestes Fiandra and Dr. Roberto Rubio. The pacemaker was manufactured by Dr. Rune Elmqvist of Elema-Schönander in Sweden, and was implanted in Uruguay in a 34-year-old patient with AV block. This unit worked successfully for nine and a half months, until the patient died of sepsis from an unrelated infection. Continue reading→
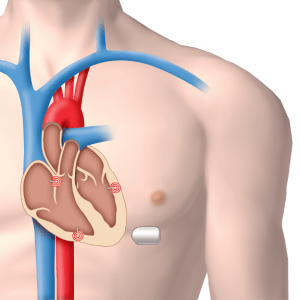 Morgan Technical Ceramics (MTC) announced that its Bedford, Ohio site manufactures the piezoelectric ceramic materials used in EBR Systems, Inc.’s innovative new WiCS® Wireless Cardiac Stimulation System. The PZT (lead zirconate titanate) material made by MTC is critical to the efficiency of the WiCS system, which is powered by a battery with a 10-year lifespan.
Morgan Technical Ceramics (MTC) announced that its Bedford, Ohio site manufactures the piezoelectric ceramic materials used in EBR Systems, Inc.’s innovative new WiCS® Wireless Cardiac Stimulation System. The PZT (lead zirconate titanate) material made by MTC is critical to the efficiency of the WiCS system, which is powered by a battery with a 10-year lifespan.
The WiCS technology, which recently began clinical trials, is the first truly wireless pacing device. It was developed to eliminate cardiac pacing leads, historically a major source of complications and reliability issues. Continue reading→
Medical Product Manufacturing News announced that ST Microelectronics is developing ultra-low-power Systems On Chip (SOCs) suitable for implantable medical devices. ST Microelectronics’ 65nm features a Vt of only 0.6V that can be used very near threshold. The REISC processor consumes barely 10.8pJ/cycle at 0.6V. This type of technology will certainly enable many new implantable devices that must operate at extremely low powers and squeeze every bit of juice out of their batteries or energy-harvesting means. Click here for link to article.
 The November issue of Evaluation Engineering carried an article by Tom Lecklider on the amount of work invested by Medtronic to develop and test the Revo MRI pacemaker system. The article is available on-line at Evaluation Engineering (Click here for direct link to the November 2011 issue).
The November issue of Evaluation Engineering carried an article by Tom Lecklider on the amount of work invested by Medtronic to develop and test the Revo MRI pacemaker system. The article is available on-line at Evaluation Engineering (Click here for direct link to the November 2011 issue).