 InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
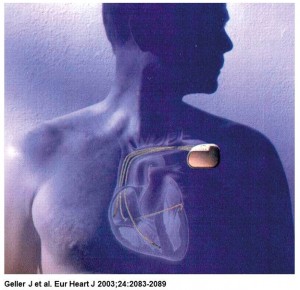
The implantable atrioverter system consisted of an implantable atrial defibrillator (model 3000 or 3020) connected to right atrial (perimeter right atrial model 7205) and coronary sinus (perimeter coronary sinus model 7109) defibrillation leads and a bipolar endocardial ventricular pacing lead, a programmer, and a defibrillation systems analyzer. The device had a volume of 53cc and weighed 79 g (model 3000) or 82 g (model 3020).
The device detected AF and delivers R-wave synchronous defibrillation shocks to convert AF to sinus rhythm. It was also able to pace the ventricle after shock delivery in case of bradycardia. Shocks could be delivered at a selected voltage up to 300V. The model 3000 defibrillator had an 80µF capacitor and could deliver a maximal shock of 3J with a biphasic waveform of 3 ms/3 ms. The model 3020 defibrillator had a 160-µF capacitor with a maximal shock of 6J with a biphasic waveform of 6 ms/6 ms. Continue reading


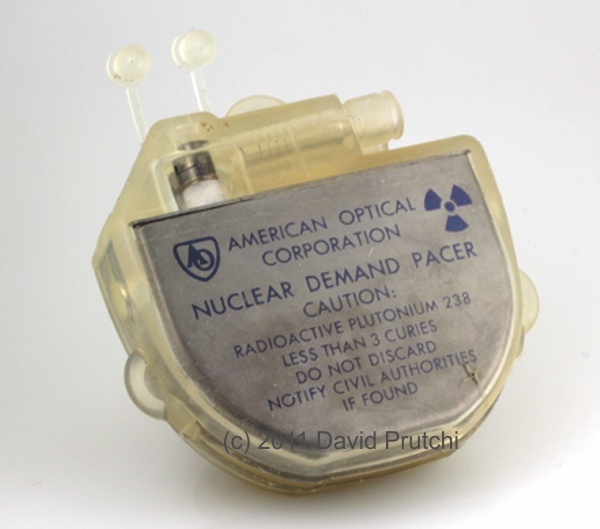


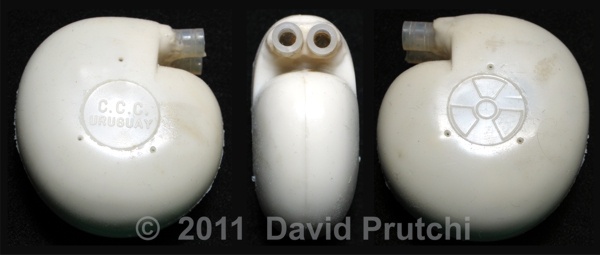


 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.

 Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.
Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005. Transoma was the name that Data Sciences International of St Paul, MN adopted in 2003 when it re-fucused its animal telemetry implant business to develop an implantable wireless system to capture electrocardiogram data for diagnosing human cardiac arrhythmias, as well as to monitor the electrical activity of the heart and transmit data from the patient’s home to monitoring centers.
Transoma was the name that Data Sciences International of St Paul, MN adopted in 2003 when it re-fucused its animal telemetry implant business to develop an implantable wireless system to capture electrocardiogram data for diagnosing human cardiac arrhythmias, as well as to monitor the electrical activity of the heart and transmit data from the patient’s home to monitoring centers. Sicel Technologies, Inc. was founded in 1999 and was based in Morrisville, NC. It ceased operations in 2010 after it declared bankruptcy.
Sicel Technologies, Inc. was founded in 1999 and was based in Morrisville, NC. It ceased operations in 2010 after it declared bankruptcy.