
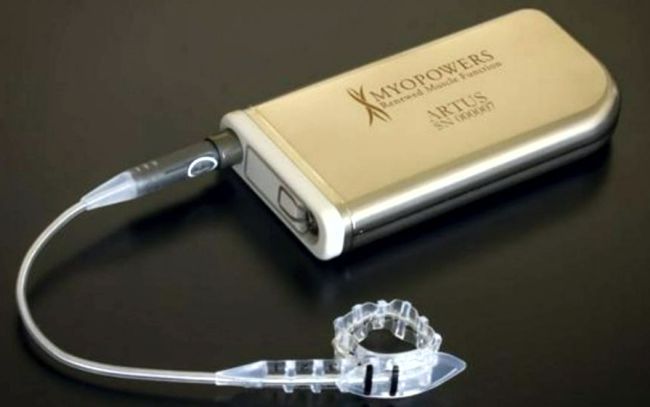
Mennen Medical’s Model 801 pacemaker. From: Dr. Shmuel Yerushalmi’s memoir at www.makash.org.il/sy/jerusa_book.pdf
I spent the year-end holidays in Israel and had the pleasure of speaking with some of Mennen Medical’s and Omikron Scientific’s first employees regarding the efforts that these companies made in the late 1970s and early 1980s towards the development and manufacture of pacemakers in Israel.
Benny Kiron, who joined Mennen Medical in 1976 as a product designer, referred me to Dr. Shmuel Yerushalmi’s memoir at www.makash.org.il/sy/jerusa_book.pdf, from which I was finally able to understand the origin of Mennen Medical’s pacemaker project, as well as its relationship with Omikron Scientific.
Dr. Shmuel Yerushalmi was born in Brasil and emigrated to Israel in 1956. In 1976, while still pursuing his doctorate at the prestigious Weizmann Institute, he started working at the Israeli subsidiary of Mennen-Greatbatch of Clarence, NY.
The Mennen-Greatbatch partnership was dissolved when Wilson Greatbatch decided to concentrate on lithium battery development, and the Israeli company became a subsidiary of Mennen Medical, where an implantable pacemaker project was started.

RF-powered pacemaker developed by the Weizmann Institute and meant for production by Elta. From: Dr. Shmuel Yerushalmi’s memoir at www.makash.org.il/sy/jerusa_book.pdf
Shmuel Yerushalmi had worked on the development of an RF-powered pacemaker while still a technician at the Weizmann institute, so his experience was tapped at Mennen Medical to lead the development of a VVI pacemaker powered by a Greatbatch lithium battery. The project was supported by Israel’s Chief Scientist Office and the BIRD (Israel-US Binational Industrial Research and Development) Foundation .

PCBA of Mennen Medical’s Model 801 pacemaker. Device was kindly gifted to me by Koby Kamin. ©2025 David Prutchi.






 I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:






 Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently
Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently  On June 5, 2013 Greatbatch, Inc. announced that it would combine Greatbatch Medical and Electrochem Solutions – which have been operating independent operations and sales & marketing groups – into singular sales & marketing and operations groups serving the entire Greatbatch organization. According to the
On June 5, 2013 Greatbatch, Inc. announced that it would combine Greatbatch Medical and Electrochem Solutions – which have been operating independent operations and sales & marketing groups – into singular sales & marketing and operations groups serving the entire Greatbatch organization. According to the