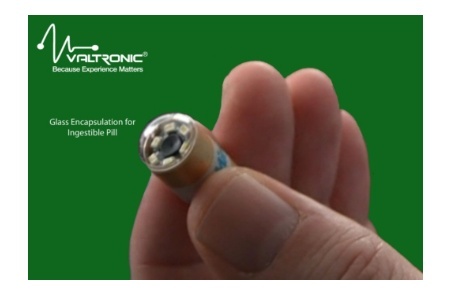
Valtronic is offering advanced glass encapsulation for active implantable devices. Their first commercial application for this product is for an ingestible pill, but this technology is suitable for many long-term implants that can take advantage of its high density feed-through reliability, intrinsic miniaturization, and radio frequency/light transparency (e.g. implantable RFID tags, sensors, microstimulators, etc.)
Valtronic is offering advanced glass encapsulation for active implantable devices. Their first commercial application for this product is for an ingestible pill, but this technology is suitable for many long-term implants that can take advantage of its high density feed-through reliability, intrinsic miniaturization, and radio frequency/light transparency (e.g. implantable RFID tags, sensors, microstimulators, etc.)
Glass encapsulation has been used in other high-tech industries and is a proven technology. Hermetic glass encapsulation provides a totally leak-proof housing for an implant. Glass-sealed packages are used mostly in critical components and assemblies solving several problems in the development and manufacture of active implants due to its strong properties and extended life.
In a recent press release, Jim Ohneck, Valtronic’s Chief Marketing Officer stated:
“This new capability provides Valtronic with a unique opportunity to reduce an implant’s power consumption and increase its functionality while reducing its size. We have exclusive rights to this technology for the medical industry worldwide and are excited about this great opportunity.”
Valtronic has released an interesting, very informative, and technically-oriented white paper about the technology. Click here for the pdf file of the white paper.