
Miniature, ultra-flexible electrode (credit: EPFL)
Aleva Neurotherapeutics is a spinoff of the Swiss Federal Institute of Technology (EPFL) Microsystems Laboratory. Aleva is developing unique microfabricated devices to more specifically target deep-brain stimulation.
kurtzweilai.net recently published an interesting blog about the technology. From the post:
“Miniature, ultra-flexible electrodes could be the answer to more successful treatment for Parkinson’s diseases, according to Professor Philippe Renaud of the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland.
He has developed soft arrays of miniature electrodes in his Microsystems Laboratory that open new possibilities for more accurate and local deep brain stimulation (DBS).


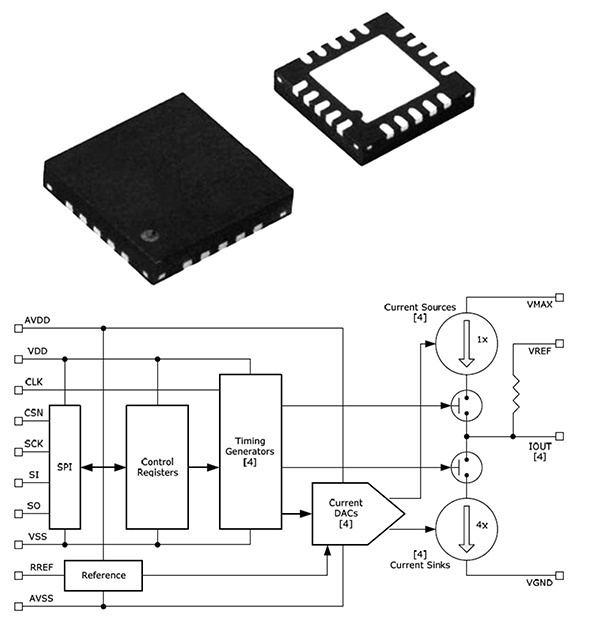







 Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.
Nanowattics was founded in 2007 to provide development services of ultra-low-power application-specific integrated circuits (ASICs) for safety-critical applications.


