
The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.
Implantable deviced developed by David Prutchi Ph.D.

The First Workshop on Active Implantable Medical Devices took place at in Punta del Este, Uruguay last Friday (March 1st 2024) as part of the LASCAS 2024 meeting.

I’ll be presenting the keynote “AIMD-based Therapies of Heart Failure” at the First Workshop on Active Implantable Medical Devices dedicated to understanding the trends and challenges in bioelectronic medicine.
For the complete announcement of the workshop with some great talks by Rikky Muller (UC Berkeley), Pedro Arzuaga (Impulse Dynamics), Marcelo Barú (Biotronik), Federico De Mula (Integer), and Victor Pikov (Medipace) click here: AIMD Workshop LASCAS 2024 Program.

Impulse Dynamics, the company for which I am the CTO, announced that FDA has approved full-body MRI-conditional labeling for the OPTIMIZER Smart Mini IPG. According to the announcement:
“The Optimizer Smart Mini system delivers the company’s proprietary CCM® therapy. This conditional approval expands the product labeling to allow for full-body magnetic resonance (MRI) diagnostic imaging with 1.5 and 3.0 Tesla (T) scanners. The approval for use with full-body MRI covers new patients adopting CCM therapy as well as existing Optimizer Smart Mini users.”

Dear Friends and Colleagues,
On October 7, 2023, Hamas perpetrated horrific acts of pure evil against innocent civilians in the south of Israel. Hamas continues to launch rockets against population centers with the sole purpose of causing terror, destruction, and indiscriminate death. These crimes cannot be justified under any circumstances.
Now, a week after the terrorist attack, over seven million of my brothers and sisters in Israel, and millions of Jews around the world live in anguish about the welfare of the hostages taken by Hamas, and concerned about the safety of those in our families who have mobilized to defend our existence.
I do not ask you to take a political stance in the Israeli-Palestinian conflict. In fact, I believe that the Palestinian People are victims of Iran and its puppet Hamas’ complete disregard for human life. Instead, I ask you to take an unequivocal stand for basic human decency against the senseless and barbaric acts of terrorism perpetrated by Hamas.
Silence is complicity!

Image credit: Impulse Dynamics
Impulse Dynamics, the company where I am the CTO and Executive VP of Product Development, announced on May 18, 2023 the completion of the first implantation for the INTEGRA-D clinical trial, designed to evaluate the safety and efficacy of two proven cardiac therapies combined — CCM® and an implantable cardioverter defibrillator (ICD) — in a single device (CCM-D). The Optimizer® IntegraTM CCM-D System delivers CCM therapy to improve quality of life and reduce heart failure symptoms, and ICD therapy to treat life-threatening arrhythmias that may cause sudden cardiac death. The investigational technology is rechargeable with long battery life, potentially reducing the need for replacement procedures.

Image Credit: Impulse Dynamics
Back on April 29, 2022, Impulse Dynamics, the company where I am the CTO and Executive VP of Product Development, announced both FDA and CE Mark approval and the official commercial launch of the Optimizer® Smart Mini System.
According to the press release:
The Optimizer Smart Mini delivers CCM therapy — Impulse Dynamics’ proprietary technology — to the heart. CCM therapy has been designed by Impulse Dynamics to significantly improve heart contraction, allowing more oxygen-rich blood to be pushed out through the body. CCM therapy is indicated to improve 6-minute hall walk, quality of life, and functional status of NYHA Class III heart failure patients who remain symptomatic despite guideline-directed medical therapy, are not indicated for CRT, and have a left ventricular ejection fraction ranging from 25 to 45 percent.
The OPTIMIZER Smart Mini offers increased battery longevity of 20 years (compared to 15 years in the previous generation), advanced HF diagnostic monitoring capabilities (e.g., heart rate, heart rate variability, patient posture and position), and remote patient monitoring features that will be activated in the near future.

On March 14, 2022, Impulse Dynamics (of which I am the CTO and Executive VP) conducted the first implant of a patient in its AIM HIGHer trial. The goal of the blinded study is to evaluate the performance of CCM Therapy to treat heart failure in a population with high ejection fraction (LVEF between 40% and 60%, inclusive).
According to the press release:
“Patients can have HF with severely reduced, moderately reduced, or even preserved ejection fraction (e.g., EF close to the normal range of 60% or above.) Those patients with severely reduced or moderately reduced EF have therapeutic options, including many classes of pharmaceuticals and device therapies such as CCM. However, HF patients with higher EF (e.g., above 40%) have had few therapeutic options thus far to alleviate their symptoms and treat their disease. The purpose of AIM HIGHer is to assess the potential of CCM to improve performance and reduce cardiovascular morbidity and mortality for these patients.
…
The Optimizer delivers cardiac contractility modulation therapy — a proprietary technology of Impulse Dynamics — to the heart. Impulse Dynamics has designed CCM therapy to significantly improve heart contraction, allowing more oxygen-rich blood to be pushed out through the body. This breakthrough device has demonstrated its capability of enhancing the quality of life for HF patients no longer responding adequately to medication meant to manage their symptoms.”
AIM HIGHer is the largest randomized sham-controlled therapeutic cardiac device trial to ever be conducted.

My new book is out and available through Amazon!
Light of specific wavelengths and intensities has been shown to repair and protect neurons from damage, opening the possibility of using near-infrared light as a non-invasive treatment of various brain and psychiatric disorders.
This book serves as a practical overview of the scientific fundamentals, technical implementation, and therapeutic applications of transcranial photobiomodulation:
This book uses straightforward language and offers practical guidance to help readers quickly develop an understanding of the practical aspects of HIPNITT implementation and its therapeutic applications.
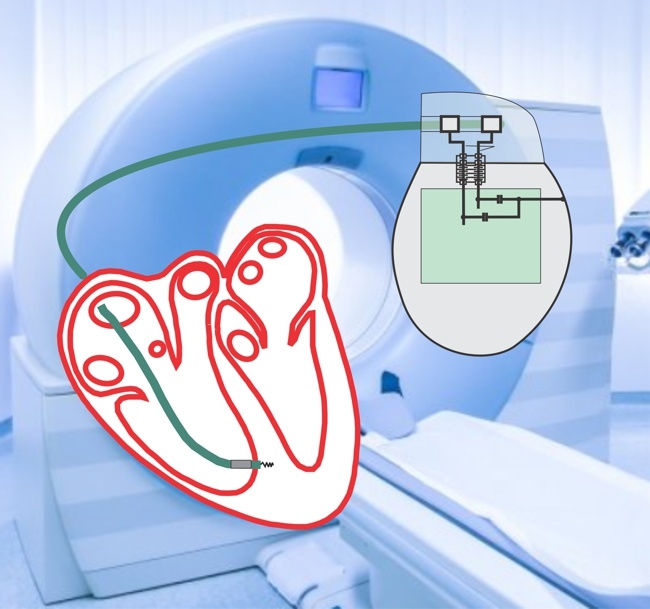
My colleagues and I presented yesterday two posters at the meeting of the International Society for Magnetic Resonance in Medicine (ISMRM). In these papers we provide support for the idea that MR-conditional leads from one system can be matched with MR-conditional IPGs from a different system without compromising safety related to RF-induced heating .
D. Prutchi, J. Meyers, R. Shehada, RF Impedance of MR-Conditional Pacemaker Leads when Connected to Implantable Pulse Generators from Different MR-Conditional Systems, ISMRM 2021, Poster 1701_2281, Paper
In this paper,we demonstrate that at 63.87 MHz the RF impedance of IPGs is minimal relative to that of the leads, which dominates the overall impedance of the implantable system. Accordingly, mixed hybrid systems composed of MR-Conditional leads and any MR-Conditional IPG are expected to have a comparable overall impedance and consequently produce the same RF-induced heating as their corresponding original systems specified by the manufacturers.
J. Meyers, D. Prutchi, R. Shehada, Input Impedance Comparison of MR-Conditional Cardiac Implantable Pulse Generators at the 1.5T MR Frequency of 63.87 MHz, ISMRM 2021, Poster 2311, Paper
In this study, we measured the impedance at 63.87 MHz of several MR-conditional IPGs from different manufacturers to assess the role of the IPG in determining the RF-induced heating at the lead electrodes. From the narrow impedance range measured, we suggest that the IPG component of an MR-Conditional system could be interchanged without compromising safety.
We also presented the following poster:
D. Prutchi, J. Meyers, R. Shehada, Importance of Pacemaker Lead Preconditioning for MR Safety In-Vitro Studies, ISMRM 2021, Poster 2282, Paper
In this study we investigated the change in the RF filtering characteristics of the leads of active implantable medical devices (AIMDs) as body fluids seep into the leads during the initial post implant period. Our findings indicate that the RF characteristics change dramatically with fluid absorption, making it necessary to precondition the leads by soaking in isotonic saline solution to simulate the in-vivo scenario when conducting in-vitro MR safety testing. Furthermore, leads designed with RF-attenuating lumped inductances must consider the effect of fluid absorption on changing the peak RF attenuation frequency.
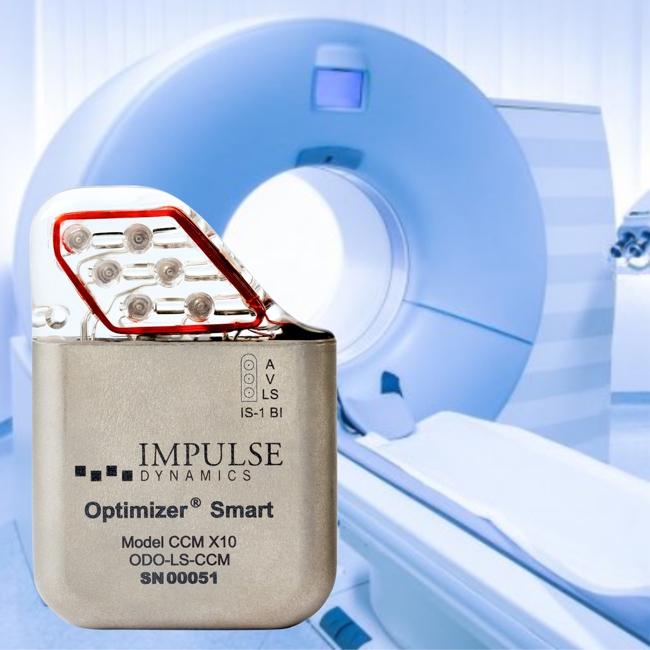
Impulse Dynamics – the company for which I am CTO and Executive VP – received MR-conditional approval (1.5 T head/limbs with peripheral coils) from both FDA and the European Union.
From the press release:
IMPULSE DYNAMICS ANNOUNCES FDA APPROVAL FOR MAGNETIC RESONANCE IMAGING FDA
Clears Potential Hurdle for Many Heart Failure Patients
MARLTON, N.J.–(BUSINESS WIRE)–Impulse Dynamics, a company dedicated to improving the lives of people with heart failure (HF), today announced the U.S. Food and Drug Administration (FDA) has approved the conditional use of Magnetic Resonance Imaging (MRI) for Optimizer® CCM® delivery systems. This approval represents a significant advance because the population that benefits most from cardiac contractility modulation therapy (patients with moderate to severe HF) often requires advanced diagnostic imaging procedures.

Impulse Dynamics (where I’m Executive VP of Product Development) announced today the completion of an $80.25 Million Series D financing round with major new investors, including strategics. According to the press release:
“Impulse Dynamics, developer of Optimizer® Smart System for delivering CCM™ therapy, announced the completion of an $80.25 million Series D financing with new investors. The proceeds will be used primarily to facilitate U.S. commercialization of the Optimizer Smart, an FDA-approved implantable device for treating chronic heart failure that has been proven to strengthen the heart and help it beat more forcibly. Led by well-respected medical technology investor Amzak Health Investors, the round also included Wellington Management, Kennedy Lewis Investment Management, Acorn Biosciences and Minth Holdings Limited; strategic investors Zoll Medical Corporation, Abiomed and one additional corporate investor; and the company’s chief executive and chief financial officers.”

Impulse Dynamics – the company for which I’m Executive VP of Product Development – announced that it has secured transitional pass-through (TPT) payment for the OPTIMIZER Smart System for the treatment of heart failure.
CMS has affirmed the substantial clinical improvement that Cardiac Contractility Modulation (CCM™ therapy) offers to patients with heart failure and adjusted the payment offered for the technology accordingly to facilitate access. The new payment, issued on Friday, Nov. 1, 2019 in CMS’s calendar year 2020 Outpatient Prospective Payment System (OPPS) final rule, eases access to CCM therapy in the hospital outpatient setting, with improved reimbursement taking effect on Jan. 1, 2020.
 Impulse Dynamics – the company for which I work – announced during HRS that a first patient received the Optimizer® Smart System for delivering CCM™ therapy since the device was approved by the U.S. Food and Drug Administration. The implant was performed on May 6, 2019 at The Ohio State University Wexner Medical Center.
Impulse Dynamics – the company for which I work – announced during HRS that a first patient received the Optimizer® Smart System for delivering CCM™ therapy since the device was approved by the U.S. Food and Drug Administration. The implant was performed on May 6, 2019 at The Ohio State University Wexner Medical Center.
The start of commercial implants in the U.S. is a major milestone for the Company. It makes me very proud that the technology on which I have worked for over 21 years is now available to make a dramatic change to the lives of heart failure patients across the U.S.
Panel: Unmet Needs Straight from the Heart of the Cardio Market
Thu. June 13| 11:00 AM – 11:45 AM | MD&M East | Medtech Hub, Booth #1669
The cardiovascular space has been among the most exciting in medtech in recent years, with advances like transcatheter procedures pushing the envelope when it comes to patient care. So where do we go from here? In this session, you’ll hear cardiologists and innovative companies in the cardiac space discuss where the next phase of innovation is in the field, what the next opportunities will be, and how engineers can move the needle.
For more information, please click here.

From Impulse Dynamics today:
FIX-HF-5C Late-Breaking Clinical Trial Data Presented at HRS 2018 with Simultaneous Publication in the Journal of the American College of Cardiology: Heart Failure
Orangeburg, New York, May 10, 2018 – Impulse Dynamics (USA), Inc. announced results from its FIX-HF-5C randomized, Controlled Trial of Cardiac Contractility Modulation in Heart Failure. The study results were presented by Prof. William Abraham, MD, Professor of Internal Medicine and Director, Division of Cardiovascular Medicine, The Ohio State University Wexner Medical Center, at the Heart Rhythm Society Late-Breaking Clinical Trials today in Boston, Massachusetts, with a simultaneous publication in the JACC: Heart Failure Journal.
CCM™ is a therapy unique to the Optimizer® family of devices that deliver electrical signals to the heart that are intended to reduce symptoms and improve exercise tolerance in patients with heart failure. The FIX-HF-5C study was designed to prospectively confirm a subgroup analysis of the prior FIX-HF-5 pivotal trial, showing that CCM™ significantly improved exercise tolerance and quality of life in patients with NYHA class III and IV heart failure symptoms, ejection fraction 25-45%, and a QRS <130ms. A Bayesian statistical analysis plan was developed to combine the data already available from the original FIX-HF-5 study with the FIX-HF-5C study data. The results presented today at HRS supplement and confirm the results from the prior study, meeting the safety and effectiveness objectives of the study.
Highlights of the results include:
- CCM™ is safe; the study met its primary and secondary safety endpoints.
- Patients receiving CCM™ showed significantly better exercise tolerance and quality of life compared to their control-group counterparts.
- The composite of cardiovascular deaths and heart failure hospitalizations was statistically significantly reduced in comparison to the control group.
- Clinical effectiveness was even greater in patients with EF >35%.
“The results of the FIX-HF-5C study exceeded my expectations by not only confirming the benefits of CCM™ on important patient-centered endpoints such as exercise capacity and quality of life, but also in demonstrating the potential for CCM™ to reduce heart failure morbidity and mortality” said Prof. Abraham. “These findings support the use of CCM™ as a breakthrough therapy for heart failure patients with moderately reduced left ventricular systolic function and narrow QRS durations, who currently have no other options for improvement.”
“I would like to thank the FIX-HF-5C Study Investigators and the Impulse Dynamics’ team for completing this important study with its very encouraging results.” said Dr. Simos Kedikoglou, Impulse Dynamics’ CEO.“We anticipate submission of the final PMA module to the U.S. FDA in June 2018.”
About the Optimizer® and CCM™ Therapy
CCM™ is the brand name for the nonexcitatory electrical pulses delivered by the implantable Optimizer® device during the absolute refractory period of the heart cycle to improve systolic contraction of the heart. The Optimizer® system has been implanted in over 3,500 patients and is currently available in Europe, China, Brazil, India and several other countries around the world. Impulse Dynamics has completed numerous clinical studies, including several randomized controlled trials. The results have been published in over 70 publications in leading medical journals. The Optimizer® is limited to Investigational use only in the United States.About Impulse Dynamics
Impulse Dynamics N.V. is focused on the development of electrical therapies for the treatment of chronic heart failure. As a global leader in cardiac medical innovation, Impulse Dynamics has operations in the United States, Europe, and Asia. For more information please visit http://www.impulse-dynamics.comImpulse Dynamics (USA), Inc.
30 Ramland Road South, Suite 204
Orangeburg, NY 10962For media enquiries
Mrs. Sharon Alon
Tel: +1-347-761-3342