Image Credit: Neural Implant Podcast by Ladan Jiracek
I don’t know how I’ve missed it, but now that I found it, I’m hooked! The Neural Implant Podcast hosted by Ladan Jiracek presents engaging conversations with the people driving innovation in neuroprosthetics, brain machine interfaces, and brain implants.


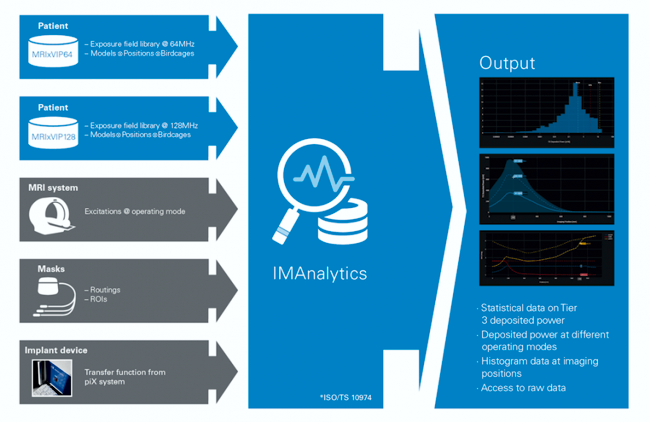








 I received today a
I received today a